
Research from data and analytics company GlobalData shows that CAR-T cell therapy innovations across APAC will make the global market for these therapies more competitive.

Research from data and analytics company GlobalData shows that CAR-T cell therapy innovations across APAC will make the global market for these therapies more competitive.
As patent disputes within the scientific community continue, drug developers consider the intellectual property unknowns associated with this emerging technology.

Emergency actions to protect patients and the drug supply may have long-term implications.

Technology and capacity investments create opportunities in the cell and gene therapy arena for CDMOs and biopharma alike.

The company has said that all three of its operating sites in China started back up on Feb. 12 and that it is closely monitoring the outbreak.
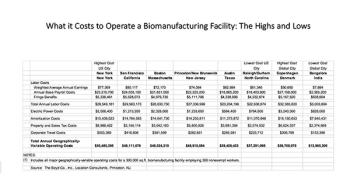
As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.

Catalent builds on its investment in cell and gene therapy development and manufacturing with the acquisition of MaSTherCell Global.

The formation of the new gene therapy company stems from the progress and success of Nationwide Children’s Hospital’s clinical manufacturing and gene therapy work.

Karen Flynn rejoins Catalent as president of biologics operations; regional presidents named for US and Europe.

The new business unit, as well as a new leadership team formed by AbbVie, will be effective upon closing of the acquisition in the first quarter of 2020.

Tim Howard will assume the role of acting ISPE CEO and president after John Bournas steps down from the positions.

The joint venture will develop next-generation cell and gene therapies incorporating Affimer proteins.

The promise of new therapies is tempered by the need for affordability, safety, and ethics.

With a positive employment market, some biopharma professionals explore options for career advancement.

The new scientific advisory board will oversee innovation projects, identify and develop key new technologies, and be responsible for forming high-caliber alliances with innovative start-ups.

Under the restructuring, Sanofi will gain sole global rights to Kevzara (sarilumab) and sole ex-US rights to Praluent (alirocumab), while Regeneron will gain sole US rights to Praluent.

Expert poll highlights strength of US pharma sector and advances by emerging markets.

Data released by the Cell and Gene Therapy Catapult (CGT Catapult) have shown that demands for specialist skills and investment in the cell and gene therapy industry in the United Kingdom are set to increase in the near future.

The innovative spirit of biotech startups is a driving force behind the development of new therapeutic products, but building a successful biopharmaceutical company from the ground up has its risks and challenges.

Nearly four decades after the first diagnosis, the fight to treat HIV/AIDs continues.

The acquisition of The Medicines Company and its investigational cholesterol-lowering therapy extends Novartis’ cardiovascular pipeline.

Catalent has named Mike Grippo as the new senior vice president, Strategy and Corporate Development, and Julien Meissonnier as vice president and chief scientific officer.

The company celebrated 65-years of providing industrial vacuum cleaning systems to the market.

Legal experts in biopharmaceutical patent law shed some light on trends and recent news.
Manufacturing differences between traditional mAb therapies and newer biotherapeutics dictate whether processes should be scaled up, scaled out, or use an alternate approach for commercial production.