Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.

Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

A Q&A with Alan Shaw of Vaxinnate. This article is part of a special section on expression systems.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.
Comparing the economic feasibility of a typical glycosylated protein.
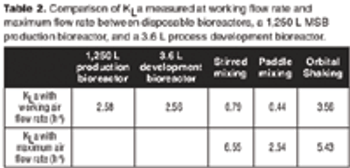
A case study to compare the performances of several types of mixing in disposable bags with stainless steel bioreactors.

A new analysis highlights growth opportunities and challenges for contract development and manufacturing organizations.
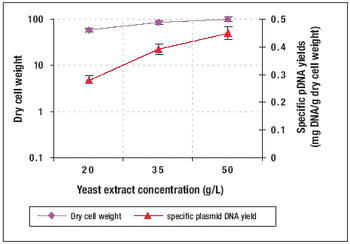
How to produce Plasmid DNA in a high-cell-density culture.
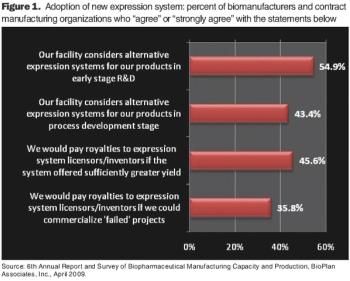
With all of the new expressions systems being developed, companies must decide what improved production and yield are really worth.

What will it take for the industry to embrace new hosts?
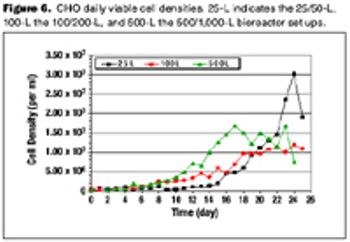
Are disposable bioreactors effective for cell culture?

With a variety of recombinant, animal-free, defined protein supplements such as growth factors, transferrin, and albumin entering the market, the biopharmaceutical industry now has innovative and safer alternatives to serum and other animal-derived supplements.
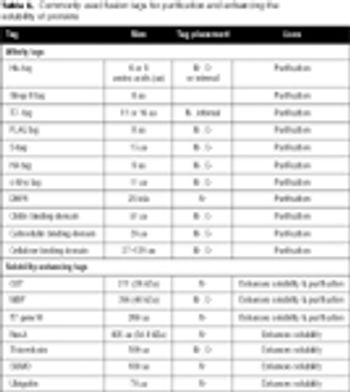
Gene fusion tags can improve the yield and solubility of many recombinant proteins. This article discusses the most popular fusion tags and the proteases used to remove them, with special reference to recently introduced technologies.
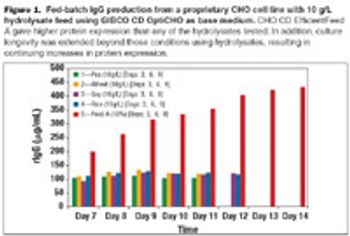
Using chemically defined feeds with CHO cell lines not only eliminates the variability associated with using plant hydrolysates, but could also improve the productivity of biopharmaceutical protein manufacture and help move therapeutic proteins into clinical trials more rapidly.
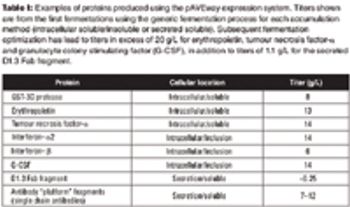
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.
Process-modeling tools can ensure smooth tech transfer.
A comparison of primary harvest techniques.

On February 21, 2008, a congressional committee sent letters to Baxter Healthcare and the FDA seeking information related to the recent heparin recall.

It is commonly believed that technologies in the next 10–15 years will enable sequencing an individualized human genome for less than $1,000. With innovations like these, the twenty-first century will certainly belong to biotechnology. From an industrial standpoint, the discovery of therapeutic molecules and the development of cell lines and processes to produce these molecules will be of paramount importance. This article describes various approaches that have been prevalent in the industry or are likely to be used in the future for generating cell lines with desirable traits and developing high titer cell culture processes.

Viragen, Inc. (Plantation, FL, www.viragen.com), and its collaborative partners in the field of avian transgenics-Roslin Institute (Scotland, UK, www.roslin.ac.uk) and (Oxford, UK, www.oxfordbiomedica.co.uk)-have announced a significant breakthrough in the development of the OVA system, an avian transgenic protein expression technology.
Growth of CHO cell cultures in a shaker plate model system was demonstrated to be comparable to growth in shake flasks.
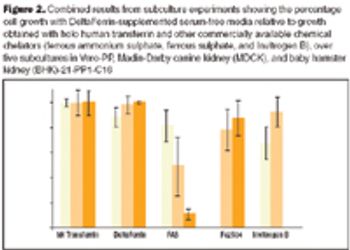
In addition to existing guidance, in January 2007 the FDA announced further proposals to prohibit the use of certain bovine materials as ingredients in some medical products or as elements of product manufacturing.
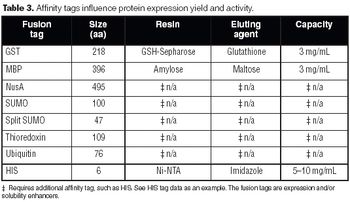
Protein expression remains an arduous task that involves a complex decision tree. Establishing tools and optimal conditions for each protein remains an empirical exercise.

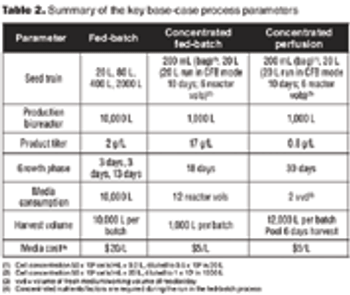
The number of biotechnology-based human therapeutic products in the late-stage pipeline, and the average cost to commercialize a biotech product, have steadily increased. This has required biotech companies to use economic analysis as a tool during process development and for making decisions about process design. Process development efforts now aim to create processes that are economical, as well as optimal and robust.
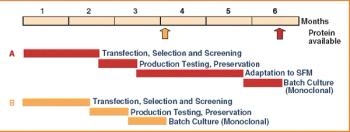
Over the last three decades, numerous protein expression systems have been developed with various quality requirements on large and small scales. Huge steps have been made in large-scale protein production in mammalian systems while the small-scale mammalian systems are expensive and inflexible. Thus, small-scale production is done in simpler expression systems, sometimes sacrificing the quality of the proteins. However, relief is on the way.