
The company has introduced two new GMP vessel streams at its API manufacturing facility in Northumberland, UK that allows it to now offer fully integrated drug substance and drug product manufacturing.

The company has introduced two new GMP vessel streams at its API manufacturing facility in Northumberland, UK that allows it to now offer fully integrated drug substance and drug product manufacturing.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

The acquisition adds sterile manufacturing capability to the CDMO’s services, which include API formulation and manufacturing.

Horizon to make publicly available its complete annotated CHO cell-line sequence in hopes of driving bioproduction cell-line innovation.

As more companies decouple drug substance from finished drug manufacturing operations, an integrated approach can ensure safe, reliable logistics for frozen storage and shipping.
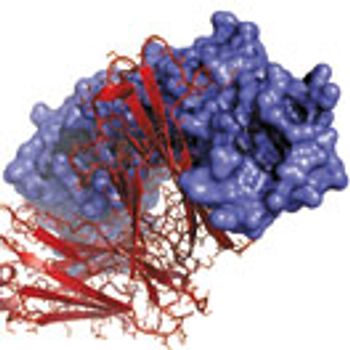
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.

The mAb is the first approved treatment that targets the progressive form of the disease.

The Basel office will house the clinical development team and other functions to progress the company’s lead compound emapalumab.

FDA granted inotuzumab ozogamicin priority review and accepted its BLA for filing.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

This three-year partnership will explore and identify new tools and methods to modify and optimize the Chinese hamster ovary (CHO) cell line performance.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.
There is much work to do to achieve efficient, cost-effective production processes.
The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Vendor selection and materials testing are complex enough, but in today’s volatile environment, risk mapping and monitoring are also crucial.

BioPharm International spoke with Trevor Marshall, director of enterprise systems integration at Zenith Technologies about automating processes in upstream processing.

Experts discuss recent advances in cell viability testing methods in bioreactors.

Results from the Phase III POLLUX trial with Janssen’s Darzalex showed that the drug was effective at reducing disease progression in patients with relapsed or refractory multiple myeloma.

The companies are collaborating on the commercialization of two biosimilar candidates in the US and Canada.

Allergan entered into a licensing agreement with AstraZeneca for MEDI2070, an anti-IL-23 monoclonal antibody in phase IIB development for the treatment of patients with moderate-to-severe Crohn’s disease.

he guidance addresses the good manufacturing practice for managing quality in APIs.