
The acquisition will give Bristol-Myers Squibb full rights to Cormorant’s HuMax-IL8 antibody program.


The acquisition will give Bristol-Myers Squibb full rights to Cormorant’s HuMax-IL8 antibody program.

In Phase III clinical trials, ixekizumab showed to be superior to etanercept and placebo in treating moderate-to-severe plaque psoriasis.

The collaboration will provide GMP manufacturing ahead of future clinical studies.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.

The agency publishes draft guidance on assay development and validation for immunogenicity testing.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.
The necessity to detach cells from a culture substrate during cell harvesting remains one of the most challenging steps in a cell-culture process.
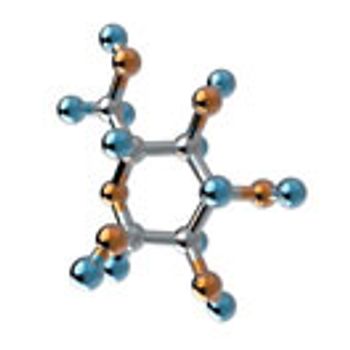
The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.

The authors describe the impact of the knocking of the pgi gene of the wild type MG1655 strain on the growth kinetics of plasmid-free and plasmid-bearing cells.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

FDA warns the industry of possible contamination in the API baclofen from Taizhou Xinyou Pharmaceutical & Chemical Co., Limited.

Use of a subspace model is a viable method to characterize process space variables and optimize process performance.

Rafe Swan/Getty Images; Dan WardIrreproducible preclinical research is a global, expensive, and well-recognized problem that contributes to delays a

The agency cited Pan Drugs Limited with improper cleaning of facilities and equipment.

Licenses could potentially bring antibody R&D to areas beyond oncology, such as diabetes treatments

The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

Catalent licenses Excelimmune’s antibody combination therapy platform toenable the manufacture of multiple recombinant antibodies in a single batch culture.

The agency cited VUAB Pharma, located in the Czech Republic, for cGMP deviations.

Choosing the optimal protein expression vector depends on strain, promoter, and a number of other factors.

Antibodies in research should be standardized and categorized using a barcode-like classification system, according to research published in Nature.

Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

The partnership will focus on the discovery of antibodies against proteins that are not easily purified in functional form.

The Cell Therapy Catapult, a UK non-profit center for advancing cell and gene therapies, will manage the manufacturing center, which will be used for late-phase clinical trials and commercial supply.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.