
This new method uses inter-alpha inhibitors to promote attachment and long-term growth in stem cells.

This new method uses inter-alpha inhibitors to promote attachment and long-term growth in stem cells.
Scale-up of complex, innovative products requires commercialization models that are sustainable.

BioPharm sat down with an intellectual property lawyer to examine how companies are protected when they engage in activities where sharing of trade secrets must occur.

A modular cell-culture platform demonstrates accelerated process development.

The novel antibody-engineering platform works differently than most of the currently employed antibody-modifying technologies, according to UM Baltimore.

Barry Holtz, Principal, at Holtz Biopharma Consulting, and Klyo Collaborative, spoke with BioPharm International about collaborative success strategies for biopharm companies.
Single-use and modular technologies plus continuous manufacturing are increasingly important to biopharma scale-up and tech transfer.

Integrating advances in facility design can meet differing and emerging bioprocessing needs.

Under an NIH contract, Paragon Bioservices will design a manufacturing process for recombinant human rhE-selectin protein.

For cellular therapies to become a viable treatment for large-sized patient groups, steps need to be taken to develop an efficient manufacturing and supply system to minimise the cost of goods.
The targeted delivery of cytotoxic drugs using antibody drug conjugates would not be possible without effective linkers to connect and then release the key chemical and biological materials.

Novasep's Sius single-use tangential flow filtration skid offers a 100% single-use TFF solution.

EMD Millipore and PharmaCell have entered into a collaboration to develop optimized large-scale expansion and harvest of HepaRG cells using bioreactor technology.

Project will optimize expansion and harvest of human cells in bioreactors.
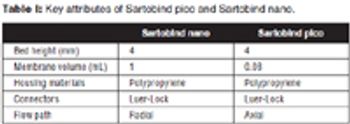
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.
Scaling up stem-cell cultures requires careful consideration of the bioreactor design.
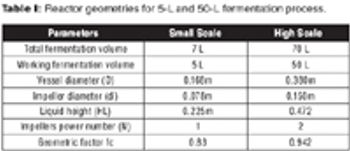
The authors present scale-up from a 5-L fermentor to a 50-L pilot-scale using the criterion of constant power consumption per unit liquid volume.
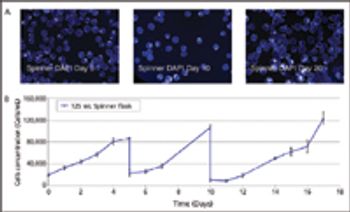
Demonstration of large-scale stem-cell scale-up.
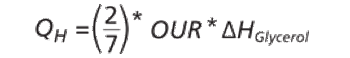
The authors describe challenges faced in transfer and scale-up of a fermentation process.

A hollow fiber matrix allows for efficient harvest of secreted proteins.
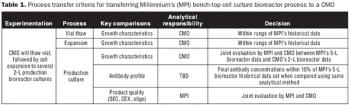
Sufficient process history is key to the rapid transfer of your process.
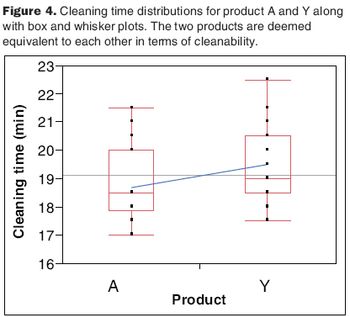
The two-one-sided t-test compares the equivalency of two data sets.
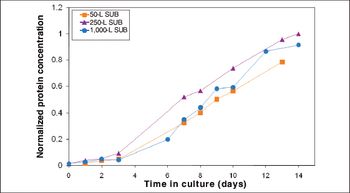
Process performance was comparable across all scales, and fiber optic sensors appeared interchangeable with conventional probes.
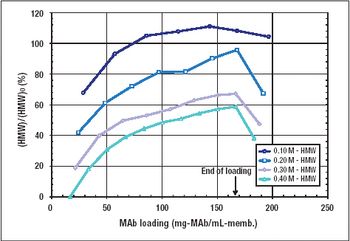
This alternative to column chromatography is suitable for flow-through as well as bind-and-elute purification operations.
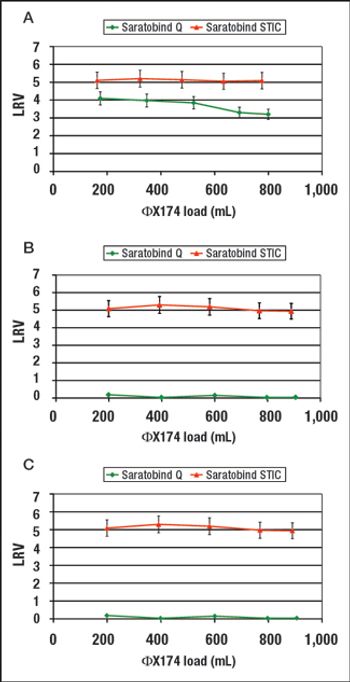
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.