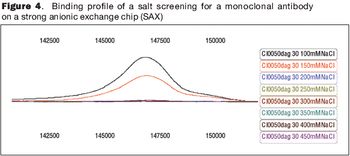
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
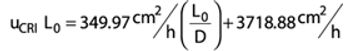
Robust packing procedures can improve process performance and increase resin lifetime.

A close-up look at Pfizer's biotherapeutics plant in Shanbally, Ireland.
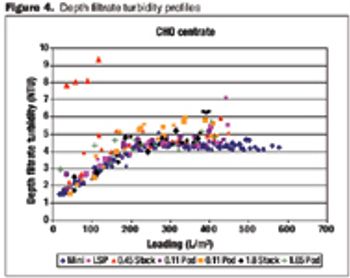
Data on the performance and variability of different formats.
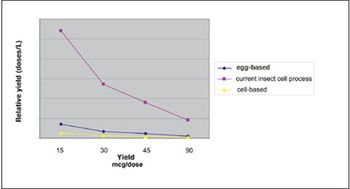
With virus-based production, vaccines can be available in 10-12 weeks.

In biomanufacturing today, there is increasing focus on improving process development. The goal is to accelerate development and reduce costs, without compromising the ability to scale up to a robust commercial process later on.

How to implement a risk-based approach to eliminating viruses.
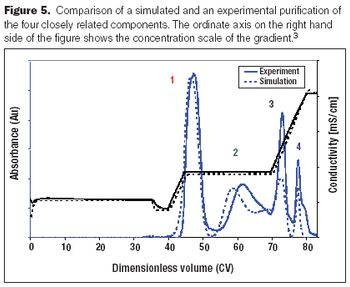
A review of some recent contributions in process chromatography.
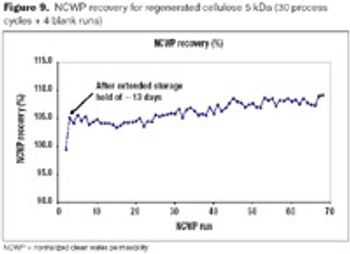
Best methods to maximize product yield and membrane lifetime to enhance a tangential flow filtration process.
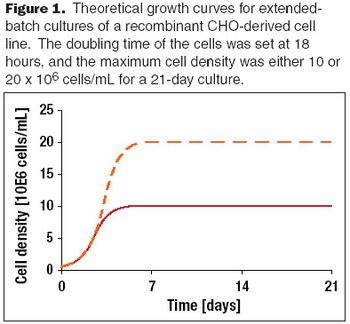
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.

With a variety of recombinant, animal-free, defined protein supplements such as growth factors, transferrin, and albumin entering the market, the biopharmaceutical industry now has innovative and safer alternatives to serum and other animal-derived supplements.
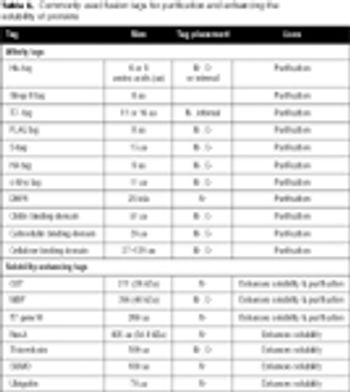
Gene fusion tags can improve the yield and solubility of many recombinant proteins. This article discusses the most popular fusion tags and the proteases used to remove them, with special reference to recently introduced technologies.
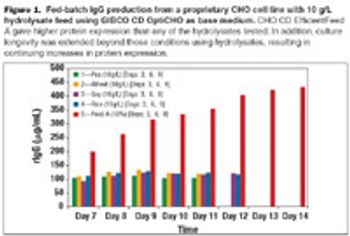
Using chemically defined feeds with CHO cell lines not only eliminates the variability associated with using plant hydrolysates, but could also improve the productivity of biopharmaceutical protein manufacture and help move therapeutic proteins into clinical trials more rapidly.
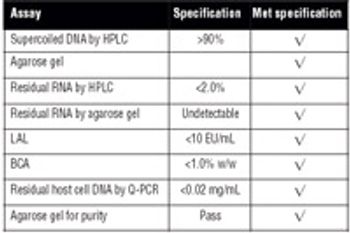
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.
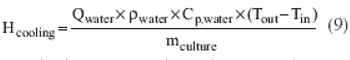
Process-modeling tools can ensure smooth tech transfer.
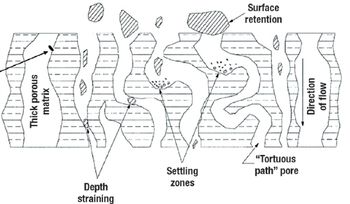
A comparison of primary harvest techniques.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted
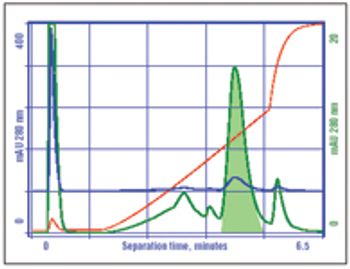
Manufacturing challenges surround the use of IgM monoclonal antibodies, but these can be overcome with current technology.
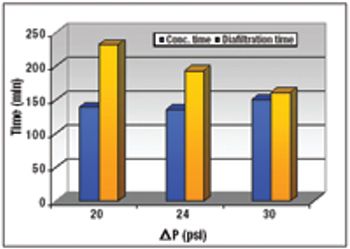
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
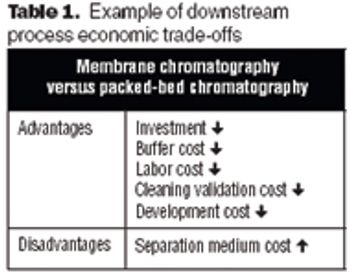
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
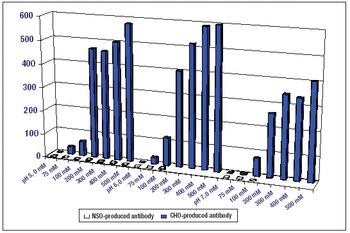
If certain engineering challenges can be addressed, precipitation may prove to be a valuable tool for antibody purification.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.

The use of disposables has changed significantly in the biopharmaceutical industry.

Design space concepts are key to a successful technology transfer.
A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.