
Experts give insight on method transfer, QbD, and regulations for analytical method development and validation for biopharmaceuticals.

Experts give insight on method transfer, QbD, and regulations for analytical method development and validation for biopharmaceuticals.

Industry players offer suggestions for quality metrics as FDA continues to try and solve the problem of drug shortages.

USP releases new and revised standards for organic impurities in medicines for public comment.

New identifiers and tracking requirements aim to block illegitimate products.

Accelerated testing and production create challenges in documenting product quality.
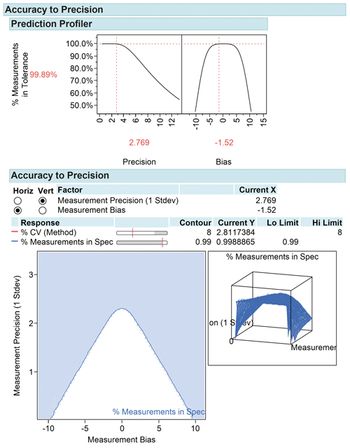
Design of experiment is a powerful development tool for method characterization and method validation.

FDA provides recommendations for submitting analytical procedures and methods validation data.

ISPE and PDA take on the challenge of recommending quality metrics.

USP opens expanded Shanghai facility to enhance quality standards for medicines and food ingredients.

Regulators work with manufacturers to improve systems for measuring product performance and manufacturing reliability.

Abrams Royal Pharmacy is recalling all unexpired lots of sterile products dispensed in the US.

The international “Fight the Fakes” campaign will raise awareness about the dangers of counterfeit medicines.

USP issues call for candidates for its 2015-2020 Council of Experts.

Using closed systems opens up many new possibilities for how facilities are designed and operated and may also present lower risk to the operation and, ultimately, the product.

Legislators agree on a limited bill affirming FDA authority over compounders while setting up a process for national drug tracking.

FDA is seeking a permanent injunction against a dietary supplement manufacturer following the company?s repeated distribution of unapproved drugs and adulterated dietary supplements.

Manufacturing standards are considered key to preventing drug recalls and shortages.

By embracing efficiency and quality, biopharmaceutical organizations can work better and achieve better work.

The authors discuss the application of risk management in process lifecycle validation, manufacturing, and change control.

ISPE study reveals quality systems of manufacturing as the leading cause of drug shortages.

USP appoints regulatory experts to elemental impurities implementation advisory group.

FDA Discovers Microbial Contamination in Compound Pharmacy Products

Ranbaxy's $500 million settlement for producing adulterated drugs and fradulent data provides a cautionary tale for patients, FDA, and drug manufacturers.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

USP defers implementation date to work closely with ICH Q3D. USP will also form a new advisory group for implementation of the new general chapters on elemental impurities.