
Developing a quality agreement template for single-use systems.

Developing a quality agreement template for single-use systems.
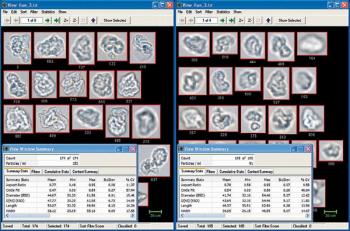
Overcoming limitations of volumetric techniques and detecting transparent particles.

US Pharmacopeia promotes horizontal standards and a product-class approach for quality attributes.

Industry may be its own obstacle to success in achieving the desired high-performance state.
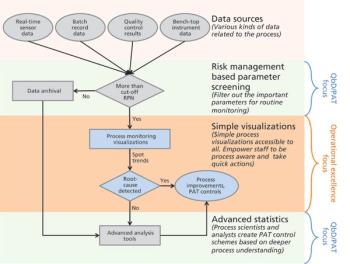
The authors focus on operational excellence in manufacturing of biotechnology therapeutic products in the QbD paradigm.

Executive management leadership is essential in the effective implementation of QbD.

US Pharmacopeia develops and improves its class approach for ensuring quality biopharmaceuticals.

The authors present an approach for testing statistical equivalence of two stability profiles.
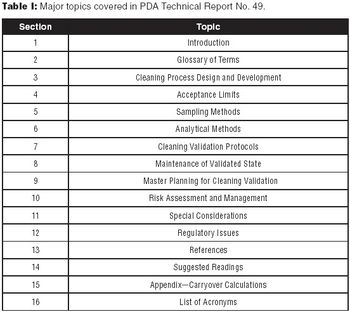
The authors encourage biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.

An approach to reduce batch time, increase productivity, and decrease costs.
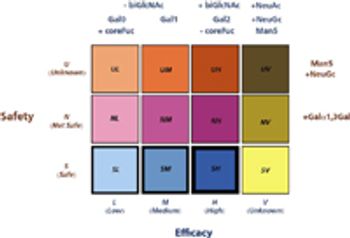
An approach to biopharmaceutical development that combines Quality by Design with a suite of visual informatics tools to reduce scale-up risks.
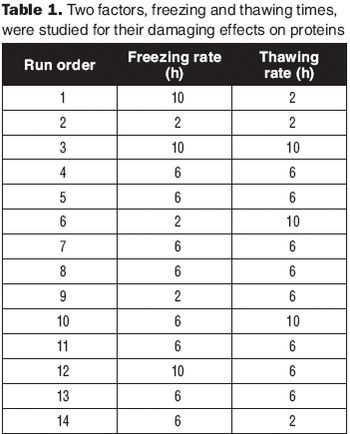
Apply a DoE strategy to test several formulations in parallel.

Best practices to strengthen supplier quality management.
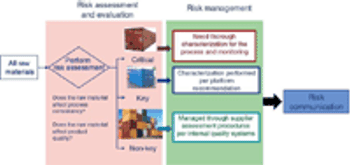
Adequate characterization of materials protects product quality.

How should the industry educate the public about product quality?

Industry and regulators disagree over noncritical parameters.

Too many REMS cause headaches for doctors and the industry.
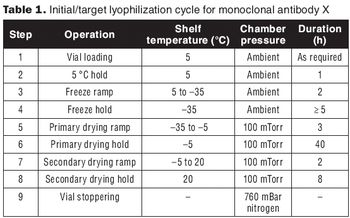
Develop a relevant design space without full factorial DoE.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.
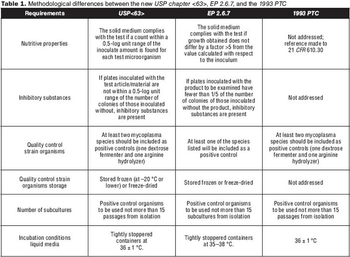
What you need to know about USP chapter <63>.
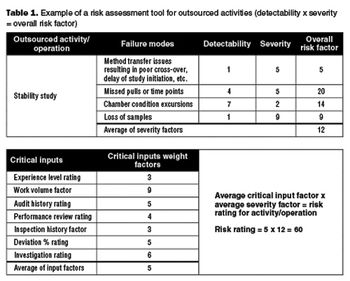
Apply risk management principles to monitor outsourced activities.

USP is advancing efforts to develop a guidance for evaluating bioassays.

Twelve lessons of what to do and what not to do to avoid quality problems.

It is important that all stages of the audit be given an equal measure of attention.
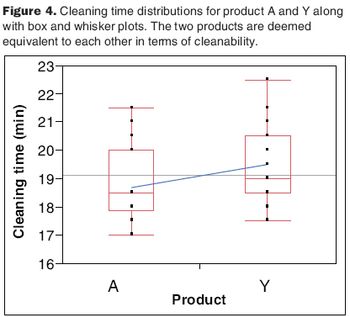
The two-one-sided t-test compares the equivalency of two data sets.