
Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

This article takes a look at current practices for cleaning and sterilizing biomanufacturing equipment used in a multi-product versus single-product setting.

The growing trend of partnerships between small biotech companies and CDMOs makes the need for conducting CMC due diligence increasingly important.
Increasingly complex monoclonal antibody molecules will require the right “tool box” for scaling up manufacturing.
Drug makers continue to explore innovative ways to develop antibody-drug conjugates based on their unique potential to neutralize cancer cells.
A look at recent investments by contract manufacturers to increase single-use bioreactor capacity.

Ireland’s National Institute for Bioprocessing Research and Training received funding from the Science Foundation Ireland’s Technology Innovation Development Award program for monoclonal antibody research.

In a deal potentially worth up to $460 million, Genentech and Xencor will develop and commercialize novel cytokine therapeutics.

MilliporeSigma’s use of modified amino acids can simplify the fed-batch process in biomanufacturing.
For rapid scale-up of biomanufacturing under expedited review status, facility design must better integrate product development and manufacturing lifecycle activities.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

The company has rolled out the first batch of TRACON Pharmaceutical’s lead product candidate from its Singapore-based single-use bioreactor.
This article will explore the requirements for media and supplements needed to maintain newer cell lines, such as those based on human cells and fungal cells.
The second half of this article describes the testing methods used by the authors to demonstrate the applicability of single-use mixing technology for virus inactivation.
This article explores the use of single-use mixing technology in a detergent-based virus inactivation step during a monoclonal antibody production process.
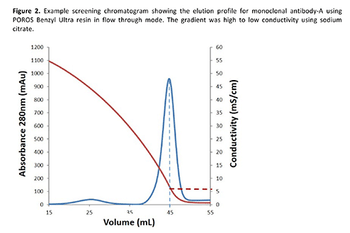
Hydrophobic interaction chromatography (HIC) in flow-through mode offers a more efficient and cost-effective polishing/purification process to remove monoclonal antibody aggregates while maintaining purity at ≥99% than a mixed-mode bind/elute procedure.
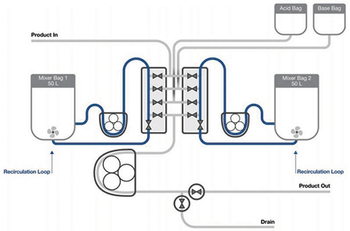
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

BeiGene is set to build late-stage clinical and commercial production capacity for cancer monoclonal antibodies with GE Healthcare’s KUBio, the prefabricated biopharma facility based on single-use technologies.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.
The quality of the cell lines used to manufacture biopharmaceuticals are crucial for the production of high-quality, stable biopharmaceuticals.

The media, by Tosoh Bioscience, is composed of calcium and phosphate buffers and offers mixed-mode properties for biomolecule purification.

Increasing demand for biologics is driving the need for innovation in bioprocessing.
Late-stage and commercial biomanufacturing pose a challenge to cell-culture processing.

The new resin used a combination of “jetting” technology and a high-performance Protein A ligand.

The contract development and manufacturing company has received an additional approval from Health Canada to manufacture monoclonal antibody drug substance at its first plant in Icheon, South Korea.