
The authors review methods for generating monoclonal antibodies for research and development.

The authors review methods for generating monoclonal antibodies for research and development.

FDA grants priority review for emicizumab, an investigational bispecific monoclonal antibody, for treating hemophilia A with factor VIII inhibitors.

With £4.5 million (US$5.8 million) in funding, the consortium is tasked with developing a new automated continuous biologics purification unit to make biologic drug manufacturing more efficient.

Horizon to make publicly available its complete annotated CHO cell-line sequence in hopes of driving bioproduction cell-line innovation.

FDA advances the progress of biosimilars with acceptance of regulatory filing for biosimilar referencing a blockbuster biologic by Roche.

Improved resin chemistries and customized separation solutions are enabling more efficient separations.
The authors present a shift toward more integrated purification processes.

Continuous processing of 100 g of monoclonal antibody in 24 hours has been demonstrated using lab-scale equipment.
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.
Including next-gen antibodies in pharma pipelines is considered essential for future success.

The mAb is the first approved treatment that targets the progressive form of the disease.

The Basel office will house the clinical development team and other functions to progress the company’s lead compound emapalumab.

Sanofi and Lonza formed a joint venture to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland.

FDA granted inotuzumab ozogamicin priority review and accepted its BLA for filing.
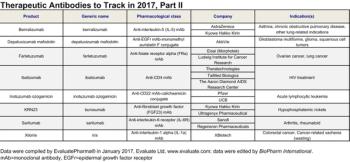
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

According to results from the FOURIER trial, Repatha significantly reduced the risk of cardiovascular events and death in patients with atherosclerotic cardiovascular disease.

The companies will combine expertise on T-cell therapies with two or more binding domains to create novel oncology medications.
The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Results from the Phase III POLLUX trial with Janssen’s Darzalex showed that the drug was effective at reducing disease progression in patients with relapsed or refractory multiple myeloma.

The companies are collaborating on the commercialization of two biosimilar candidates in the US and Canada.

Allergan entered into a licensing agreement with AstraZeneca for MEDI2070, an anti-IL-23 monoclonal antibody in phase IIB development for the treatment of patients with moderate-to-severe Crohn’s disease.
An approach to stabilize PBS-based formulations could provide a simple physiological solution for use of proteins in research, preclinical, diagnostics, and clinical studies, as well as commercial biotherapeutic products.