
Large-scale implementation of Protein A chromatography offers several challenges.

Large-scale implementation of Protein A chromatography offers several challenges.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

Antibodies in research should be standardized and categorized using a barcode-like classification system, according to research published in Nature.

The potential blockbuster treatment targets a protein involved in cholesterol homeostasis.

Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

The partnership will focus on the discovery of antibodies against proteins that are not easily purified in functional form.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.Roche Acquires Bispecific Antibody Developer Dutalys

Amgen's bispecific T-cell engager (BiTE) antibody constructs help the body fight malignant cancer cells.

Ranbaxy and Epirus announce the launch of India's first biosimilar for Remicade.

Opdivo and investigational agent FPA008 will be tested in combination for their efficacy in boosting antitumor immune response.

The final guidance explains some principles for developing biosimilars and establishes some rules about extrapolation across indications for various medical conditions.

Celgene expands its oncology drug discovery and development portfolio through a new partnership with Sutro.

A Priority Review voucher Sanofi and Regeneron purchased from BioMarin pharmaceuticals may put their mAb ahead of Amgen's in the market.

Personalized immunotherapy treatments help 90% of acute lymphoblastic leukemia patients achieve remission in a new study.

Single-domain antibodies are emerging as credible alternatives due to their target specificity, high affinity, and cost-effective recombinant production.
Establishing the CQAs of a mAb product by evaluating impact and uncertainty during risk assessment.

Different approaches to prepare highly concentrated feed media for fed-batch Chinese hamster ovary cell culture are evaluated.
The approval and acceptance of monoclonal antibody biosimilars is necessary if the biosimilars market is to experience real growth.

A prototype Protein A resin is evaluated for purification performance, reusability, and cost performance.
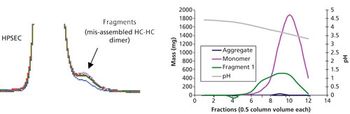
The authors discuss the evolution of the purification platform for manufacturing of mAb therapeutics.

The EMA's Committee for Medicinal Products for Human Use has recommended granting of marketing authorizations for the first two monoclonal antibody biosimilars.
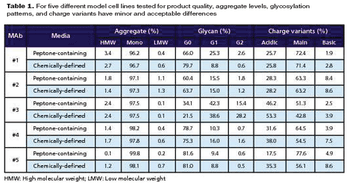
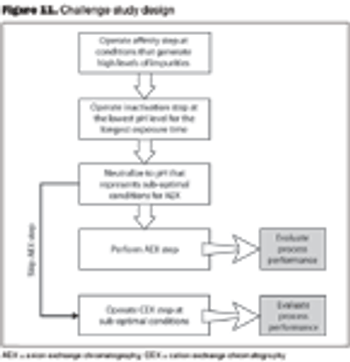
How to use risk assessment strategies to integrate operations.