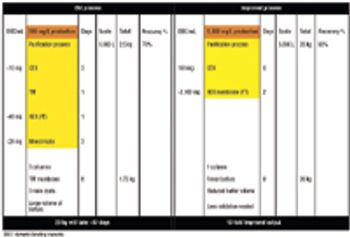
Affinity purification schemes for antibody production have certain limitations keeping up with cell culture expression levels as they reach and exceed 10 g/L. New downstream purification processes are based on low cost, long lasting, and high binding (40–100 mg/mL) cation exchange resins.