
A new facility to be built in the Philadelphia Navy Yard will support commercial production for autologous tumor-infiltrating lymphocyte cell therapy products from Iovance Biotherapeutics.

A new facility to be built in the Philadelphia Navy Yard will support commercial production for autologous tumor-infiltrating lymphocyte cell therapy products from Iovance Biotherapeutics.

The expansion will increase the company’s single-use capacity to meet growing demand.

Catalent expands gene therapy capabilities with $1.2-billion acquisition of Paragon Bioservices.

The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

The partnership will focus on the commercialization of inducible promoters to improve biomanufacturing.

The new 40,000-ft2 facility will support Bayer's growing biologics portfolio in oncology, cardiology, and additional therapeutic areas.

Vibalogics has increased its single-use bioreactor and purification capacity with a new manufacturing for its specialist oncolytic virus and viral vector manufacturing services as a result of a growth in demand.
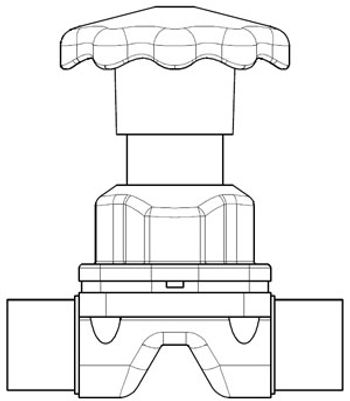
Valve design and materials affect performance and cost to maintain.

Recent upstream processing innovations include enhanced sensor technology, single-use bioreactors, and automated cell culture systems.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

This article takes a look at current practices for cleaning and sterilizing biomanufacturing equipment used in a multi-product versus single-product setting.

The company passed a seven-day FDA surveillance GMP inspection and announced two upcoming manufacturing partnerships.

At INTERPHEX 2019, CEO Richard Johnson highlighted the importance of PDA’s new Asian business unit and outlined the organization’s plans. Data integrity guidance for manufacturing and quality systems will be published by the end of the year, as efforts move into big data and artificial intelligence.

The growing trend of partnerships between small biotech companies and CDMOs makes the need for conducting CMC due diligence increasingly important.
Increasingly complex monoclonal antibody molecules will require the right “tool box” for scaling up manufacturing.

More manufacturers are embracing MAM, which simplifies biopharmaceutical product quality testing, and facilitates the measurement and monitoring of critical quality attributes.

The company’s Quantum peristaltic pump uses a patented single-use cartridge technology and is applicable to downstream bioprocessing.

The companies form a strategic joint venture for developing and manufacturing live biotherapeutics.

ITT Engineered Valves presented its new EnviZion hygienic valve technology at INTERPHEX 2019 in New York City.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Adding to an existing $115-million investment, the 700,000-ft2 site in Longmont, CO will expand AveXis’ gene-therapy manufacturing capacity.

A look at the Univercells' NevoLine bio manufacturing platform, which incorporates principles of automated and continuous bioprocessing.

Close collaboration between academic and industrial groups is vital to ensuring glycosylation models are fit for deployment.
Drug makers continue to explore innovative ways to develop antibody-drug conjugates based on their unique potential to neutralize cancer cells.

NJII and Pall collaborate to address biomanufacturing technology and workforce development challenges.