
Lonza plans to invest approximately CHF 850 million (US$936 million) to build new state-of-the-art mammalian manufacturing facilities at its Visp, Switzerland, and Portsmouth, NH, sites.

Lonza plans to invest approximately CHF 850 million (US$936 million) to build new state-of-the-art mammalian manufacturing facilities at its Visp, Switzerland, and Portsmouth, NH, sites.

AkesoBio’s expanded manufacturing capacity, enabled by the addition of another Cytiva FlexFactory platform, will be used to meet current and future market needs in China as well as worldwide.

The new facility, which will be Evotec’s first in Europe, will aim to offer capacity, flexibility, and quality for biotherapeutic development and manufacturing while utilizing technology that employs small, automated, highly intensified, and continuous bioprocessing operations inside autonomous cleanrooms.

Catalent’s new mammalian cell culture suites in Madison, Wis. use single-use bioreactor systems for CGMP clinical and commercial manufacturing.

The China National Medical Products Administration has given the company the green light to begin manufacturing commercial supply of its approved anti-PD-1 antibody, tislelizumab, at its biologics facility in Guangzhou, China.
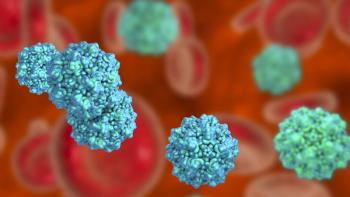
A series of minute virus of mice spiking studies were performed with a monoclonal antibody product and small-scale AEX membrane adsorbers to evaluate a range of critical processing conditions.

The transaction is set to close during the first half of 2021 and is expected to enhance drug substance and drug product capabilities for WuXi.

Overcoming time and cost constraints can help enable seed train intensification efforts to maximize product yield.
While manufacturing increases have been implemented for vials and syringes, meeting future market needs remains unpredictable.

Lessons learned from the pandemic can advance future biologic drug development and manufacturing.

The CGMP manufacturing agreement will expand production of lenzilumab, a candidate for COVID-19 therapy, to support a potential emergency use authorization filing.

Using rDNA technology to synthesize production of proteins and peptide hormones, the biotech startup has achieved synthetic production of insulin, potentially lowering insulin cost by 30%.
Cell-culture optimization may see benefits from a synthetic biology-based approach that improves product titer, quality, and time.
The development of an innovative purification process simplifies downstream processing for biologics.

The grant will support the advancement of glycoengineering technology for plant-derived proteins developed by a University of Alberta scientist partnered with PlantForm.

Wacker will support production of CureVac’s COVID-19 mRNA-based vaccine candidate at its biotech site in Amsterdam, with production scheduled to start in the first half of 2021.

The new super plant, being built in Incheon, South Korea, will have a total biopharmaceutical manufacturing capacity of 256,000 L.

The companies have formed a long-term manufacturing agreement to accelerate the global supply for Lilly’s COVID-19 antibody therapeutics.

The agreement follows a recent announcement that Sartorius plans to invest $100 million in Songdo, South Korea’s bio cluster.

Sanofi’s Digitally Enabled Integrated Continuous Biomanufacturing Facility in Framingham, MA was named the Overall Award winner at the ISPE virtual annual meeting.

Biopharma can apply new manufacturing practices adopted during the COVID-19 pandemic to enhance bioprocessing.

The agreement will provide capacity for the manufacturing of AZD7442, currently being developed for the potential prevention and treatment of COVID-19, at Lonza's Portsmouth, NH site.

Continuous SEC was shown to increase productivity with the same product quality and yield.

The shift to single-use technologies is driving the need for innovation in PAT-friendly sensor technologies.

The new VirusExpress lentiviral production platform increases dose yields and reduces process development time for cell and gene therapies.