
Bormioli Rocco Pharma offers new packaging solutions for powdered oral drugs, parenteral drugs, pediatric syrups, and protein-based drugs.

Bormioli Rocco Pharma offers new packaging solutions for powdered oral drugs, parenteral drugs, pediatric syrups, and protein-based drugs.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.
Manufacturers introduce innovations in glass and plastic packaging for injectables.

Bormioli Rocco Pharma, an Italian supplier of glass and plastic packaging, has launched its Delta-molded, type 1 glass vials in North America.

Choosing a suitable material for fill/finish containers begins during the product development stage.
Protecting against microbiological contaminationover the whole manufacturing process grows increasingly important.

An FDA evaluation concluded that Corden Pharma Latina’s corrective actions addressed the concerns in an FDA warning letter.

Ajinomoto Althea’s Optima VFVM 7000 aseptic fill/finish line supports a range of drug substance APIs.

Corning and Gerresheimer have been working together to accelerate innovations for pharmaceutical glass packaging.

A Merck, Pfizer, and Corning collaboration resulted in development of Corning Valor Glass for improved drug storage and delivery and will create US jobs.

The companies are partnering to provide new fill/finish facilities and solutions for biopharmaceutical products.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.
The author discusses the impact of prefilled syringe product contact materials and the filling and stoppering process on protein aggregates.
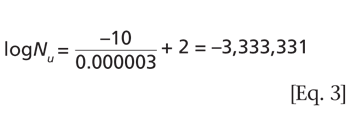
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

Tony Pidgeon, process technology director at Patheon discusses challenges associated with and recent innovations in the fill/finish process for biopharmaceuticals.

The ready-to-fill packaging solutions for vials are based on Ompi EZ-fill packaging design.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

The company expanded fill/finish services at its facility in Kalamazoo, Michigan.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses how to ensure sterility when manufacturing small-scale parenteral batches.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

Excipient selection strongly influences lyophilization performance for biologic drugs.

PMMI, The Association for Packaging and Processing Technologies, released a report on trends opportunities in packaging of pharmaceuticals and medical devices that predicts increased spending on capital equipment for processing and packaging.

IDT Biologika will provide fill/finish services of live-vectored virus products in clinical development through licensure.

Prefilled syringes for commercial supply of the etanercept biosimilar will be produced at Catalent’s Brussels facility in Belgium.