
How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

Genzyme (Cambridge, MA) will outsource fill-and-finish operations for its drugs Cerezyme, Fabrazyme, Myozyme, and Thyrogen to Illinois-based Hospira, Inc.

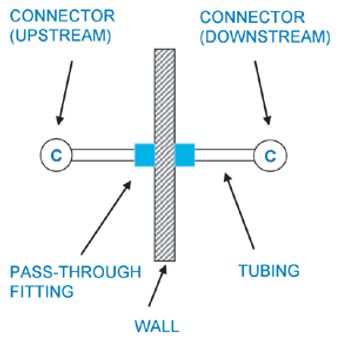
Many factors must be considered when choosing a sterile connector for a given process.
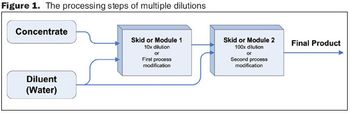
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

Protalix BioTherapeutics (Carmiel, Israel) said the US Food and Drug Administration has asked the company to consider submitting a treatment protocol for the use of prGCD, the company's development drug for patients with Gaucher disease, to address an expected shortage of Genzyme's Cerezyme. Genzyme recently halted production of Cerezyme to sanitize its plant in Allston Landing, MA, because of virus contamination of a bioreactor.
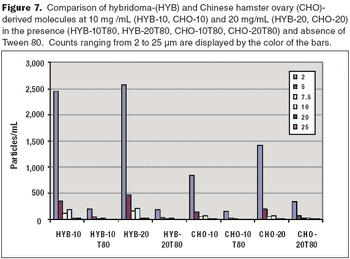
How to maintain product stability and prevent particulates.
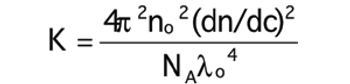
Can increase in ionic strength result in higher viscosity?

The European Medicines Agency (EMEA) has granted license approval to Bayer HealthCare LLC (Bayer, Berkeley, CA) for its new sterile filling facility on its Berkeley, CA, campus.

Boehringer Ingelheim (BI, Ingelheim, Germany) has received FDA approval for the contract manufacture of an unnamed biological drug in glass prefilled syringes.


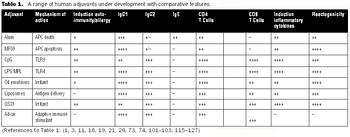
Adjuvant-caused vaccine reactions are one of the most important barriers to better acceptance of routine prophylactic vaccination.
Drug delivery technologies have the potential to enable drug candidates with poor pharmaceutical or biopharmaceutical properties, both for macromolecule and traditional compounds. While there have been many success stories to date, the future offers even more promise. In this article, the author surveys the top ten areas in drug delivery looking forward.
The many benefits of disposable technologies, such as significant savings in time, labor and capital, as well as ease of scalability and flexibility, have led to the growing trend of adopting disposable technologies in bioprocess manufacturing processes.

Formulations for pulmonary inhalation comprise spherical, porous particles that are 1–3 microns in diameter.

Disposables require less space than conventional equipment, and they can be assembled offsite into complete process trains.

A symposium at the AAPS National Biotechnology Conference in Boston, June 19-22, 2006, addressed key concerns and new developments in manufacturing biologic products in a sterile environment.

On August 12, 2003, Johnson & Johnson began recalling certain batches of its anemia drug, Eprex (epoetin alfa, sold as Procrit in the US), in most countries outside of the United States.

Biopharmaceutical products are subject to stability problems distinct from traditional sterile pharmaceutical processing and thus require more care in handling and preservation than do classical "small-molecule" drugs. Most biopharmaceutical formulations are aqueous, and protein products have limited stability in their liquid state.