
Automation and disposables continue to reduce human error.

Automation and disposables continue to reduce human error.

Drug quality issues have forced the National Institutes of Health to shutter its in-house facility for producing clinical supplies for certain clinical trials.

FDA cites 17 observations including air handling, quality control, and deficient microbial monitoring at NIH’s Clinical Center Pharmaceutical Development Section.

This article reviews factors that affect protein stability at different steps of the product manufacturing process and strategies to minimize their impact on product quality.

Removal of microorganisms is crucial when working with biologics. Sterile filtration offers a reliable, safe, and effective way to ensure product integrity.
Second-generation needle-free injection systems will make parenteral drug administration more convenient, efficient, and safe.

Bausch’s second generation Type 529 is a semi-automatic IV bag filling and closing machine designed for aseptic packaging of infusions, parenteral drugs, and other fluids with a fully integrated automatic closing system to eliminate possible contamination.

Genentech plans to invest more than $125 million in an expansion of its fill/finish facility in Hillsboro, OR.

McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

Evaluating the assembly design process, manufacture, and use helps mitigate risk.
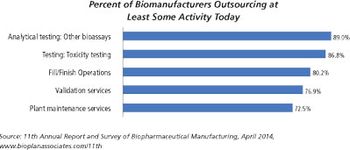
There are significant differences between small molecules and biologics fill/finish capacity.


Grand River Aseptic Manufacturing has announced that the company executed two commercial production contracts in one day.

IDT Biologika completes large-scale biologics finishing facility in Dessau, Germany.

PSC Investments announces the acquisition of a high potency, sterile fill/finish pharmaceutical manufacturing facility.

Merck KGaA fill-finish expansion in Italy will be completed in 2017.

Integrated Single-Use Sterile and SIP Connector Provide Flexibility

Aseptic Valve Range Increases Sterility

Displays will include new products for anticounterfeiting, labeling, and compliance packaging and new equipment for filling, aggregating, and quality control.

New injection-delivery systems with multiple closure points pose challenges for container closure integrity testing.

A novel approach to sterile drug product manufacturing uses a single-use assembly in a multi-product final filling suite with isolator technology.

FDA clarifies recommendations for injectable drug products packaged in vials and ampules.

Manufacturers are taking measures to comply with new package safety rules.

A novel approach to sterile drug product manufacturing that uses a single-use assembly in a multi-product final filling suite with isolator technology offers benefits of efficiency and flexibility.