
FDA sent a warning letter to an Illinois compounding pharmacy for violations of the Federal Food, Drug, and Cosmetic Act.

FDA sent a warning letter to an Illinois compounding pharmacy for violations of the Federal Food, Drug, and Cosmetic Act.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

A company’s fill/finish individual facilities can be negatively impacted by a region’s economic climate, as currently observed at some of the pharmaceutical manufacturing locations in Puerto Rico.

Industry experts discuss the challenges of using single-use systems in biopharma manufacturing.

CPhI Pharma Awards seek nominations for excellence in biopharma development and manufacturing.

Material compatibility, material sourcing, facility layout, and training are crucial aspects of a successful disposable fill-finish system.

The FlexPro 50, from Groninger, is a modular filling and closing system designed to process vials, cartridges, and syringes, as well as vials in bulk and trays.

Austrianova completes facility for GMP cell banking and fill/finish services for cell therapy products.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Two experts discuss best practices to achieve acceptable sterility assurance levels for aseptically filled products.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

Althea is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates, in a new facility near San Diego, CA.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.

Novo Nordisk will build a local manufacturing plant for FlexPen prefilled insulin delivery devices in Iran.

Preventing contamination requires quality systems to be in place, including routine cleaning, a robust cleaning validation program, and preventive maintenance.

President Obama will nominate Rob Califf, current deputy commissioner for medical products and tobacco, as FDA commissioner.
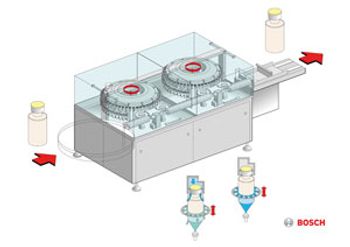
The RAN 3080 exterior washing machine from Bosch cleans filled and closed vials, ampuls, and cartridges using a high-pressure process and special transportation system.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

Three case studies illustrate some analytical methods important for stability testing.

The new, still unnamed biosciences arm of Emergent BioSolutions will be a separate public company with a focus on novel therapeutics in immuno-oncology.

Process maps and risk assessments are among the valuable tools operators can apply to reduce the risk of microbial contamination.

Industry experts discuss challenges, trends, and innovations in fluid handling.

Baxter has initiated a voluntary recall of two lots of IV solutions due to the potential presence of particulate matter.