
Advances in fill/finish for parenteral packaging address demands for efficiency and product safety.

Advances in fill/finish for parenteral packaging address demands for efficiency and product safety.

Optima Pharma opened a new assembly hall, the CSPE Center, that will give the company space to build complete, multi-story pharmaceutical manufacturing facilities.

Nephron Pharmaceuticals is partnering with Clemson University to create a robotic solution for syringe-filling automation to enhance sterile manufacturing.

Catalent recently held a groundbreaking ceremony at its Bloomington, Indiana pharmaceutical fill/finish site.

Lyophilization Services of New England acquired a sterile injectables manufacturing facility in León, Spain.

CDMO iBio introduced cGMP sterile fill/finish services at its facility in Texas.

Sterile filtration is often required for biologics but presents degradation and compatibility challenges.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.
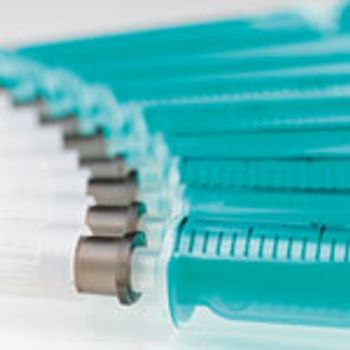
Both empty and filled syringes must pass a range of tests to meet quality standards for biopharmaceutical drugs.

The company will open its first European manufacturing facility in Castlebar, Ireland.

The Flexicon FPC60 peristaltic fill/finish system from Watson-Marlow Fluid Technology Group can use a variety of modules that allow users to create their own customized filling solution to suit small-batch applications.

To ensure the sterility of parenteral biopharmaceutical products, it is necessary to employ certain tools, technologies, and standard operating procedures.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.

The sterile-manufacturing contract development manufacturing organization is approved by FDA for viral vector manufacturing fill/finish processing at its biologics facility in Scotland, UK.

Pfizer will invest nearly half a billion dollars to build a sterile injectable facility in Michigan.

Single-use technologies have become increasingly prevalent in final fill/finish operations for biologics.

Aseptic fill/finish is crucial in biopharma manufacturing and is optimized through automated technology.

Rentschler Fill Solutions and Ultragenyx announce fill and finish agreement for the US commercial supply of Mepsevii.

In adding a Vanrx Pharmasystems aseptic filling isolator, FUJIFILM adds fill/finish for gene therapies and viral vaccines.

RGtimeline/Shutterstock.comParenteral product quality is improving.

The author reviews operative options for the implementation of a quality oversight and how companies can benefit from this function from a regulatory perspective.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

Ompi EZ-fill vials and Daikyo Seiko PLASCAP press-fit closures are a confirmed product set for use with Vanrx Pharmasystems' Aseptic Filling Workcells.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.