
Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.

Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.

Capacity for complex therapeutics is become increasingly difficult to predict.

Biopharmaceutical manufacturers continue to put quality at the forefront of their relationships.

Brammer Bio establishes late-phase development and commercial manufacturing facility for advanced cell and gene therapies in Lexington, MA.

The new company is the product of a merger with Formex.

Contract biopharmaceutical manufacturing has been growing steadily and is expected to reach $4.1 billion by 2019.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

Changes in China’s Food and Drug Administration (cFDA) drug development and commercialization policies make it easier for multinationals and CMOs to manufacture in China for in-country use, reports CMO and consultant PaizaBio.

Better process development is creating industry benchmarks for bioprocessing.

Althea is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates, in a new facility near San Diego, CA.

BioPharm International eBooks

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.
Focusing on niche and specialty service offerings gives contract biomanufacturing organizations an opportunity to differentiate in a crowded market.

CMC Biologics and River Vision Development announce manufacturing agreement for RV001, a monoclonal antibody to treat Grave’s orbitopathy.

While the United States and Europe still dominate, CMOs and CROs based in emerging markets continue to capture market share.

Rentschler Biotechnologie launches 2000-L single-use bioreactor and announces additional expansion.

The fast growth of the global biopharmaceutical market has prompted global pharmaceutical and biotechnology companies to increase their R&D investment in biologics.
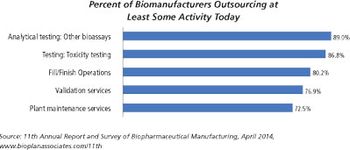
There are significant differences between small molecules and biologics fill/finish capacity.

A dynamic market, industry consolidation, and demand fluctuations lead to a mixed picture of pricing results.

Representatives of contract service organizations discuss technology trends with BioPharm International.

The author highlights the top 10 outsourcing trends found during a survey of biopharmaceutical manufacturers.

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Successful management of the CMO/client relationship should include open communication and trust.
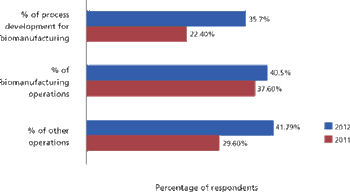
Will international biomanufacturing outsourcing become mainstream in this decade?

The manufacturing capacity-sharing model between Merck and MedImmune ushers in a new paradigm of "co-opetition."