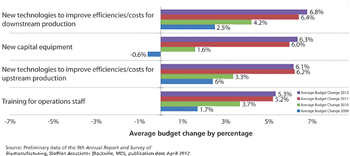
Industry optimism is on the rise for 2012.

Industry optimism is on the rise for 2012.

Industrializing design, development, and manufacturing of therapeutic proteins.

China rises to the top as a destination for international outsourcing.
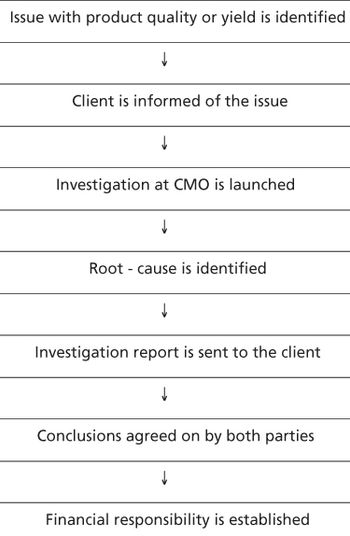
The authors present the CMO's perspective on various potential sources of sponsor-CMO conflicts.
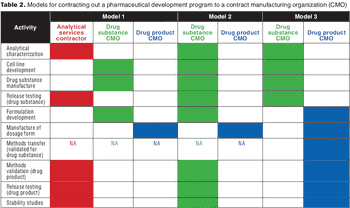
Formulation strategy is an important consideration when selecting and managing outsourced biopharmaceutical development programs.

Partner with a contract manufacturing organization that integrates best practices from project management, customer service, and Lean.

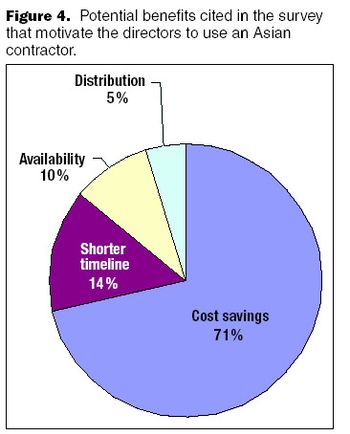
Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.
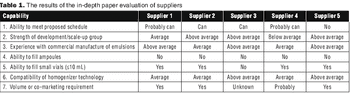
"A systematic approach taken by a company involved making an assessment of internal capabilities, strengths, and needs before the selection process."

How to ensure smooth technology transfers.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.

After a strategic evaluation of what activities to outsource, sponsor companies should follow key guidelines for selecting and auditing a provider and preparing quality agreements.
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

A CMO faces significant risk of lost revenues and profits if the product fails in clinical trials or doesn't meet sales projections.

China's pharma industry represents 5% of the world total. By 2010, China is expected to become the world's fifth largest pharmaceutical market.

Cobra Biomanufacturing is an international full-service manufacturer of biopharmaceuticals, dedicated to designing robust processes that deliver biopharmaceutical products to its life sciences customers for preclinical through Phase 3 studies.

Discovery Laboratories (Warrington, PA) could see the end of its struggle to launch its Surfaxin (lucinactant) drug on the US market soon, as the manufacturing issues it has faced have been resolved.

There wasn't much of a contract services industry when BioPharm International began publishing 20 years ago. Today's big names in biomanufacturing, including Lonza, Boehringer-Ingelheim, and Avecia, had not yet entered the business.

From the earliest days of the biotechnology industry, companies have grappled with the complexities of making innovative biopharmaceuticals on a large scale. Success in manufacturing begins with process science, since biotech production requires perfection in maintaining living organisms in a sterile environment under controlled physiological conditions. But unless companies can solve the challenge of planning for and managing manufacturing capacity, they will not be able to achieve the full potential of promising biotech products.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) issued a revised draft guidance on July 20 to help ensure that the safety, purity, and potency of biologics products is not compromised as a result of innovative, flexible manufacturing arrangements.

Eden Biodesign (Liverpool, UK, www.edenbiodesign.com), SAFC (St. Louis, MO, www.sigmaaldrich.com/SAFC/Pharma), Midatech Group (Oxfordshire, OX, www.midatechgroup.com), Cellexus Systems (Cambridgeshire, UK, www.cellexusbiosystems.com), and BioConvergence LLC (Bloomington, IN, www.bioc.us) are sprucing up their product development and services with the construction of new manufacturing facilities.

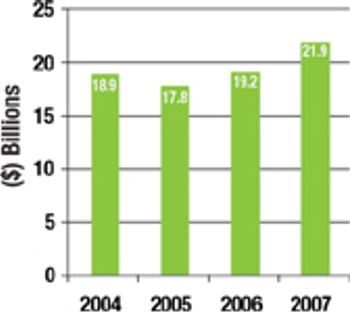
Partnering with a surging number of CROs, CMOs, CSOs, and other niche providers, biopharm companies in 2007 will have an estimated spend of more than $7 billion on international clinical trial outsourcing alone.

The contract manufacturer must have sufficient capacity so it can absorb possible surge in demand, and back-up capability in case of a power failure or other event.

The three largest players have accumulated, or are in the process of accumulating, nearly a million liters of capacity between them.

The changes in biologics manufacturing regulations contained in the 1997 FDA Modernization Act significantly bolstered the growth of CMOs.