
The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.
A different perspective on controlling fixed costs of biomanufacturing, based on know-how from other industries, provides a competitive edge, says the CEO of Samsung BioLogics.

The contract development and manufacturing organization has entered it first manufacturing contract worth $148 million for its recently completed Plant 3 facility.

Avid Bioservices will provide commercial manufacture of an enzyme replacement therapy by Roivant Sciences' Enzyvant subsidiary.

Unlike current approaches where bioconjugation is typically done following the manufacture of the monoclonal antibody and the cytotoxic drug, the new method begins with the antibody supernatants and eliminates the need for extensive chromatographic purification.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

Paragon Bioservices will build a new cGMP facility in Maryland and expand its existing cGMP facility at the University of Maryland's BioPark.
Recent investments show expansion activity in cell culture facilities.

The company has completed development of a first-generation production process for its chimeric antigen receptor regulatory T cell product portfolio and is selecting a CMO for clinical supply.

Abzena has entered into a Master Services Agreement with a US biotech company to provide process development and manufacturing services to progress a novel antibody-drug conjugate (ADC) to clinical trials.

The European Medicines Agency has granted Samsung BioLogics approval to manufacture a monoclonal antibody at the company’s second facility in Songdo, Incheon, South Korea.

The new 30,000-L, $150-million biologics manufacturing facility in Wuxi, China, quintuples the company’s existing manufacturing capability.

Biopharma majors are among the industry stakeholders who have commented and raised questions about FDA’s recently proposed draft guidance for analytical assessment of similarity in biosimilars.

Lonza has entered into strategic license agreements for exclusive rights to a gene-therapy platform for developing treatments for hearing and balance disorders.
The Biomanufacturing Technology Roadmap is accelerating innovative manufacturing strategies for biopharmaceuticals.

Biocad and Sothema Labs have partnered to release cancer-treating biosimilars into the North African market.

The acquisition gives GE Healthcare access to a nanofiber-based platform purification technology that can offer improvements in biopharmaceutical productivity.

A new facility type integrates next-generation mobile cleanroom systems.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.

Hitachi Chemical Advanced Therapeutics Solutions will expand its PCT service platform for cell therapy by adding GMP manufacturing and cleanroom capacity in Allendale, NJ.
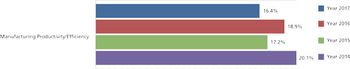
Innovation speeds discovery, drives down costs, and improves productivity.

Despite some progress, the industry is still in a wait-and-see mode regarding the administration, Congress, and FDA.

The Bridgewater facility will provide a range of contract services for biopharmaceutical and pharmaceutical manufacturers, and also serve electronics chemicals suppliers.

Samsung BioLogics signs $55.5 million agreement to manufacture tildrakizumab for Sun Pharma.