
The CDMOs are joining together to offer end-to-end development and manufacturing services for protein expression systems and viral vectors in Europe.


The CDMOs are joining together to offer end-to-end development and manufacturing services for protein expression systems and viral vectors in Europe.

Under a new alliance, KBI Biopharma and Argonaut Manufacturing Services will combine their strengths to offer end-to-end biopharma development and CGMP manufacturing solutions.

Charles River’s off-the-shelf rep/cap plasmids are intended to simplify gene therapy supply chains.

Andelyn Biosciences has been selected by Ultragenyx to manufacture UX111, that company’s gene therapy for treating Sanfilippo Syndrome.

Following the divestiture of its Elusys subsidiary and other non-core assets, NightHawk Biosciences will transform into a pure-play large-molecule CDMO.

RoslinCT will manufacture Vertex Pharmaceuticals’ CRISPR-based gene therapy, Casgevy, recently approved for treating sickle cell disease and β thalassemia.

PharmTech Europe discusses technology that enables the “democratization” of mRNA manufacturing with Scott Ripley, general manager, Nucleic Acid Therapeutics and Precision Nanosystems, Cytiva, at the 11th International mRNA Health Conference in Berlin, Germany.

Demand for efficient tech transfer, as well as compliant on-time delivery, is rising.

CDMOs are actively exploring and leveraging both new and existing technologies to streamline the cell-line development process at every step.

The new centralized hub will provide advanced testing of nucleic acids, which is expected to simplify mRNA substance testing.

As part of a $30 million investment, Aragen is setting up a new biologics manufacturing facility in Bangalore, India.

A look at using best practice tech transfer methods for CGTs to increase process and analytics robustness while being scalable.

Under an expanded agreement, Cellares will provide proof-of-concept manufacturing for a second CAR-T cell therapy from Bristol Myers Squibb.

The 65,000-square-foot facility is designed with the capacity and capability to help scale the next generation of CGTs for human trials and beyond.

Samsung Biologics and Bristol Myers Squibb have further expanded their strategic manufacturing agreement to include the large-scale manufacture of an antibody cancer drug substance.
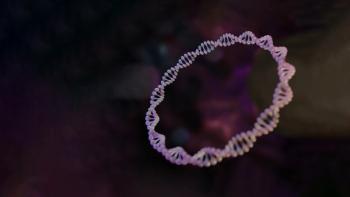
Plasmid DNA (pDNA) is a critical raw material for the manufacture of cell and gene therapies. As this market continues to grow, so too does the demand for high-quality pDNA.

Recipharm will provide analytical and process development capabilities to support toxicology studies, in addition to GLP manufacturing of lipid nanoparticles to capture the active pharmaceutical ingredient, an antigen peptide.

Rentschler Biopharma’s ATMP business can now offer its full range of services for the clinical supply of AAV, including bioprocess and analytical development through to cGMP manufacturing at the Stevenage facility in the UK.

Rentschler Biopharma, CGT Catapult, and Refeyn aim to use automated and digital technologies to improve AAV manufacturing for gene therapies.

WuXi XDC will provide end-to-end services to support Boostimmune’s discovery of novel bioconjugates.

More than a third of CMOs are struggling to keep skilled technical and production staff.

WuXi Biologics is expanding its manufacturing capacity for drug substance and drug product at its sites in Leverkusen and Wuppertal, Germany.

Hanns-Christian Mahler and Andrea Allmendinger from ten23 health will discuss some key aspects of biologic drug development and manufacturing.

uBriGene Biosciences will acquire Mustang Bio’s Worcester, Mass., CGT manufacturing facility in a deal worth up to $11 million, expanding its operations into the US market.

Synthego has opened its new GMP-compliant RNA manufacturing facility in Redwood City, Calif., for CRISPR-enabled genomics therapeutics.