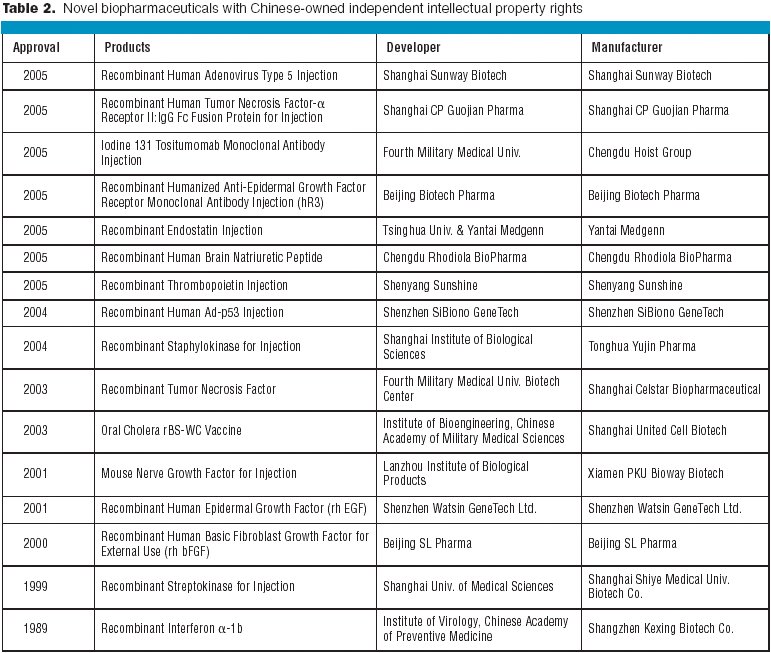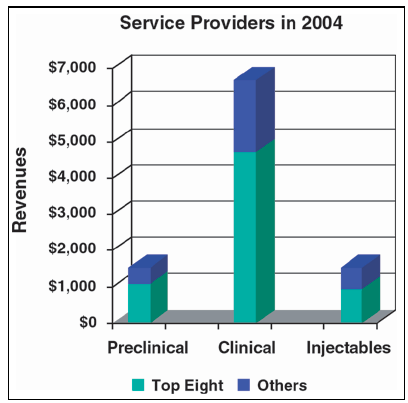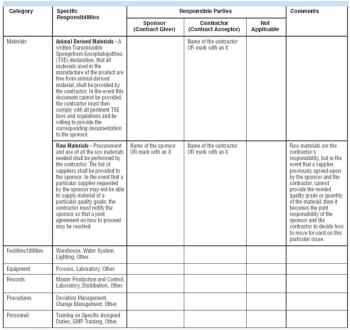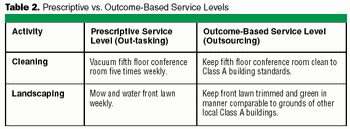
Your company's job is to make biopharmaceutical products. Managing facilities is a function supporting the main task. General manufacturing companies discovered this long ago, but pharmaceutical producers have been lagging. Once you consider the outsouring of non-core activities like facility management (FM), office services, space planning, and utilities management, you can focus on core business functions that make profits.