
The agency cited the company’s Kansas facility with CGMP violations similar to problems found at other Hospira facilities.

The agency cited the company’s Kansas facility with CGMP violations similar to problems found at other Hospira facilities.

The new company links and manages aspects of treatment delivery, facilitating access for patients to treatments by serving as a connector between manufacturers, patients, physicians, and payors.

FDA plans to initiate its quality metrics program as industry continues to push back.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

Sanofi and Lonza formed a joint venture to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland.

Thermo Fisher Scientific will provide cryo-TEM for small-molecule and biologic drug discovery.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

Sarepta sold the priority review voucher for Exondys 51, a controversial treatment for Duchenne Muscular Dystrophy, to Gilead.

In 2016, FDA approved 630 ANDAs and tentatively approved 183 ANDAs, the highest number to date, according to the report.

Metrohm USA presented the Young Chemist Award to Aldin Malkoc for his work on cooperative, DNA-based molecular elements for electrochemical biosensors.

The company opened a new bioconjugation unit to support the clinical and commercial manufacturing of ADCs.

The company announced that no Form 483 was observed at the company’s facility in Bangalore, India.

The companies have developed a Level 4 traceability solution to manage pharmaceutical regulatory requirements.

FDA granted inotuzumab ozogamicin priority review and accepted its BLA for filing.

CellGenix will add R&D, production, and warehouse space in Freiburg, Germany for GMP-grade raw materials for cell therapy, gene-therapy, and tissue-engineered products.

In a press release, Momenta announced on Feb. 16, 2017 that Momenta/Sandoz’s fill/finish contract manufacturer, Pfizer, received a warning letter for the manufacture of the 40 mg of Glatopa (glatiramer acetate injection), Momenta’s generic version of the drug Copaxone. Pfizer said in the statement that the warning letter does not affect the production or shipment of the 20 mg version of the drug, which is already approved by FDA.

The regulatory agency rejected the medication, citing various issues related to device use.

Under the agreement, Abzena will manufacture magacizumab, an antibody created using the ‘Abzena inside’ Composite Human Antibody technology.

Sartorius and EMBL have entered into a corporate partnership program to foster advanced training.

The ICH Q11 Q&A discusses the development and manufacture of drug substances and the selection and justification of starting materials.

PhRMA submits comments to the The Office of the United States Trade Representative encouraging protection of US innovation in foreign markets.

FDA approved Valeant’s brodalumab with a boxed warning for suicidal ideation.

The Patent Trial and Appeal Board ruled in favor of the Broad Institute, allowing the company to keep patents for their CRISPR-Cas9 gene-editing technology.

FDA sent a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found deviations in the facility’s aseptic processes.

South East Asian pharma manufacturers seeking region growth expected to attend CPhi South East Asia.

The directorate says monographs are flexible and changeable and their compliance does not on its own determine biosimilarity in biosimilars.

The company has voluntarily recalled all lots of of human chorionic gonadotropin because of a lack of sterility assurance.

The agency sent a warning letter to Resonance Laboratories Pvt. Ltd. after an inspection found possible contamination problems.
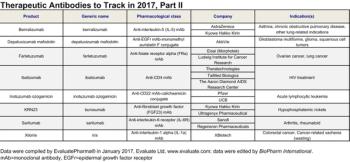
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.