
Good project management, budgeting, planning, and clear documentation are the only ways to prevent overruns and project failure.

Good project management, budgeting, planning, and clear documentation are the only ways to prevent overruns and project failure.

Communication and taking the time to develop the process are key to successful transfer and scale up of biologics

Plant transient expression offers a flexible approach in drug development and enables rapid production and scale up of therapeutics.
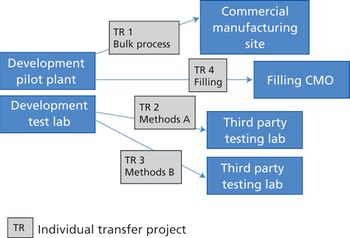
Careful planning, adequate staffing levels, and experienced project managers can help avoid pitfalls of transferring processes from one facility to another.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.
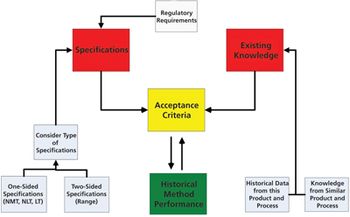
The authors present the results of a survey of biologics manufacturers to evaluate how these manufacturers transfer analytical methods.

Shire requests a technical transfer from Sanquin to widen its manufacturer base for the production of Cinryze.
Single-use and modular technologies plus continuous manufacturing are increasingly important to biopharma scale-up and tech transfer.

Ties between the biotechnology industry and university research are crucial.
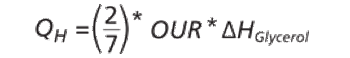
The authors describe challenges faced in transfer and scale-up of a fermentation process.
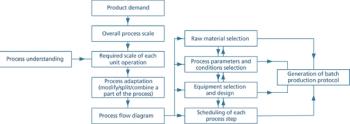
Clear documentation and open communication are essential for effective technology transfer.

The authors present lessons learned from a case study of the transfer of a cell culture biotherapeutic process to a CMO.
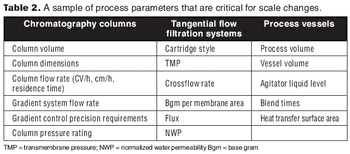
A process harmonization assessment can aid in smooth technology transfer by comparing data across equipment and sites.
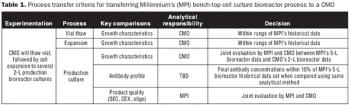
Sufficient process history is key to the rapid transfer of your process.

By following key strategies, companies can reduce the risk and increase the benefits of outsourcing analytical development and testing
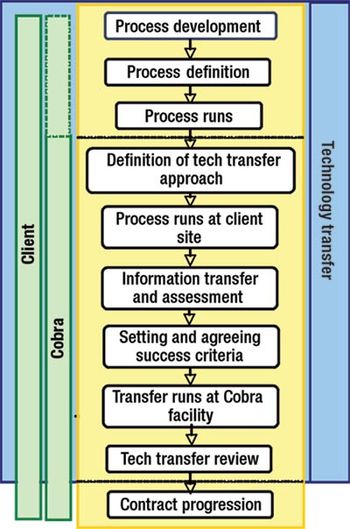
Effective tech transfer can save time and effort in later manufacturing processes.

How to ensure smooth technology transfers.

To succeed in a pandemic, the industry must forge a preparedness plan to ensure adequate vaccines.
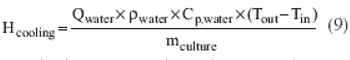
Process-modeling tools can ensure smooth tech transfer.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.
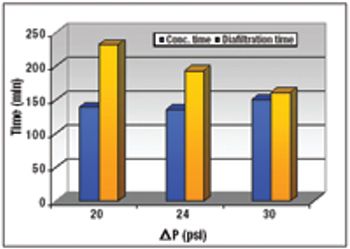
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
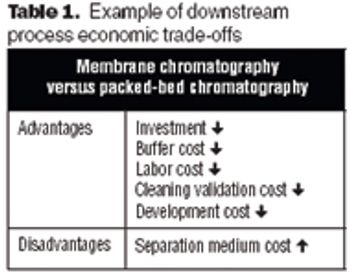
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.
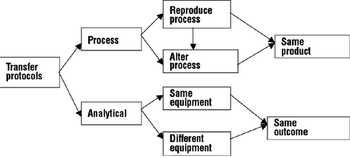
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

If a company wants to reduce costs, it should consider outsourcing some manufacturing and analytical testing to low-cost sites.