Quality/GMPs
Latest News


Manufacturers seek gradual rollout of more targeted FDA quality metrics program.

The Reagan-Udall Foundation for the FDA (RUF) is overcoming initial roadblocks and gaining support from a range of public and private organizations.
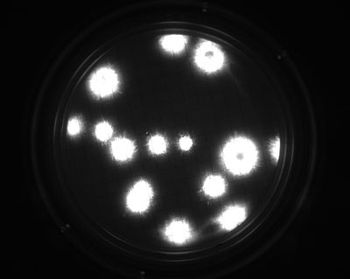
The rapid microbiological growth-based method represents an alternative for the quantification of contaminants in filterable products.

Biopharmaceutical company BeiGene plans to build facility in Suzhou to expand its clinical capability and prepare for commercial manufacturing.

Regulatory officials and industry scientists participated in a CMC Strategy Forum sponsored by CASSS in July 2015.

*This article is an opinion piece and does not necessarily represent the views of BioPharm International.

The company adds a few new arguments-as well as new stakeholder support-to its Citizen Petition on biosimilar naming.

The company is in discussions with Europe’s Adaptive Pathways Group on clinical trials that would evaluate its PLX cells for the treatment of specific diseases.

FDA determined the celebrity’s endorsement of Duchesnay’s morning-sickness medication Diclegis was misleading.

The agency issues guidance for companies considering registering with FDA as an outsourcing facility.

European Union regulators said Pfizer must divest its infliximab biosimilar candidate and some sterile injectables for the merger with Hospira to be completely approved.

FDA issues draft guidance on dissolution testing for immediate-release solid oral dosage forms.

FDA issues guidance regarding fees for drug compounding outsourcing facilities.

Process maps and risk assessments are among the valuable tools operators can apply to reduce the risk of microbial contamination.

FDA and industry support global framework and collaborations to secure the supply chain.

This article discusses cleaning validation of equipment dedicated to the production of a single API.

The broader aim of FDA's metrics initiative is to encourage quality manufacturing operations that will help avoid drug shortages.

FDA’s quality metrics draft guidance details the types of data the agency plans to request and the quality metrics it plans to calculate.

EMA’s revised guideline on the implementation of accelerated assessment is open for public consultation.

The guidance provides recommendations for submitting analytical procedures and method validation data to FDA.

FDA warns an Arkansas compounding company that it is in violation of the FDCA.

The bill would authorize spending of approximately $8.1 billion on transit projects through 2015 while legislators plan a long-term bill that could have implications for the pharmaceutical industry.

A year-long process reauthorizing the Prescription Drug User Fee Act was launched July 15, 2015 with a public meeting, setting the stage for discussions with industry and FDA.

FDA releases a report that analyses why some diseases are lacking treatment options.