
On Jan. 27, 2016 FDA announced it plans to review Merck’s investigational antitoxin prevention bezlotoxumab.

On Jan. 27, 2016 FDA announced it plans to review Merck’s investigational antitoxin prevention bezlotoxumab.

Baxter has voluntarily recalled lots of IV solutions due to potential container leakages and particulate matter.

The European Directorate for the Quality of Medicines & Healthcare announces the publication of a chemometric methods chapter in the European Pharmacopoeia.

FDA’s Center for Drug Evaluation and Research publishes a list of upcoming new and revised guidance documents for 2016.

FDA issued a warning letter to Chan Yat Hing Medicine Factory for CGMP violations.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.

Abbott’s Compounding Pharmacy voluntarily recalls sterile products due to lack of sterility assurance.

Three associated centers in California, New York, and Florida are cited for not following proper sterilization and validation procedures and for not having the appropriate licenses to manufacture a biological drug product.

A US district judge entered a consent decree of permanent injunction on behalf of FDA, between the United States and pharmaceutical company, Downing Labs.

The Office of Prescription Drug Promotion issues all-time low number of violation letters in 2015.

The company has voluntarily recalled one lot of magnesium sulfate in water for injection because of incorrect labeling.

FDA issues a Warning Letter to Cadila Healthcare Limited for cGMP violations.

Ionis Pharmaceuticals receives orphan drug designation for HTTRx for the treatment of Huntington’s disease.

More “me-betters” and more focused breakthroughs could enhance new drug development.
mstay/Getty ImagesBiopharmaceutical manufacturing involves a series of complex unit operations linked together to provide high-purity, biologic actives with specifi

The bio/pharmaceutical industry will face increased scrutiny of product quality and cost drivers.

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.
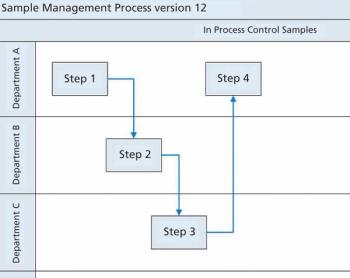
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

The agency has published draft guidance on safety assessment for investigational new drug application safety reporting.

The agency has launched a new web platform to foster scientific innovation.

FDA warns the industry of possible contamination in the API baclofen from Taizhou Xinyou Pharmaceutical & Chemical Co., Limited.

EMA Executive Director Guido Rasi outlines his plan for the agency, including a focus on R&D.

Changes in China’s Food and Drug Administration (cFDA) drug development and commercialization policies make it easier for multinationals and CMOs to manufacture in China for in-country use, reports CMO and consultant PaizaBio.

The United States Pharmacopeial Convention announced the recipients of the 2015–2016 Global Fellowship Awards.