
The agency is enlisting members of European pharmaceutical associations and industry representatives to participate in IDMP task force.

The agency is enlisting members of European pharmaceutical associations and industry representatives to participate in IDMP task force.

The draft guidance contains policies for drugs that are processed with additional manufacturing steps such as remixing, dilution, and repackaging.

FDA announced that it approved Lenvima to treat patients with differentiated thyroid cancer after it was submitted under a priority review and orphan drug programs.

The agency releases five draft guidance documents related to drug compounding and repackaging.

FDA gives an update on currently approved Risk Evaluation and Mitigation Strategies.

The National Institute for Health and Care Excellence released an updated version of its biosimilar approach guidance, including increased consideration for technology appraisal, references in documentation, and the production of “evidence summaries”.

The Center for Drug Evaluation and Research seeks a more flexible system for assessing biotech product quality.

The industry reacts to the departure of Commissioner Margaret Hamburg.

On February 3, 2015, the FDA published a notice in the Federal Register that it is soliciting input on the collection of data to support interchangeability claims in biosimilar applications.

FDA has approved Genetech’s Lucentis to treat retinopathy in patients with diabetic macular edema.

The agency releases guidance documents on Individual Patient Expanded Access Applications and disclosing risk information to patients.

Margaret Hamburg announced on Feb. 5, 2015 that she will step down as FDA Commissioner after serving in the position for almost six years.

FDA releases a Q&A with the Drug Shortage team leader from the Center for Drug Evaluation and Research.

The cyclin-dependent kinase 4/6 inhibitor for the treatment of metastatic breast cancer was approved more than two months ahead of the prescription drug user fee goal date.

President Obama unveils his “Precision Medicine Initiative”.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.

FDA says it is “weighing the appropriate regulatory approach” to handle the tasks outlined by President Obama’s new Precision Medicine Initiative.
As ADCs move through the drug-development process, different analytical methods are often required.

Initiatives to speed drug development must pass Congress and special interest groups.

Manufacturers face new rules for tracing drugs through the supply chain and compounders face stricter standards.
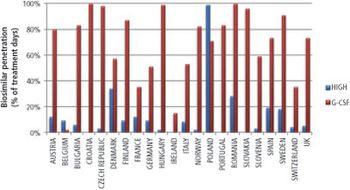
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.

EMA is under pressure to exert even tighter standards on biosimilars being marketed in Europe.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.

Developing intellectual property standards for biological products is a point of conflict as negotiations on the Trans-Pacific Partnership continue.

Bristol-Myers Squibb and Johnson & Johnson announced FDA-approval of Evotaz and Prezcobix, combination HIV-1 infection treatments.