
FDA’s breakthrough drug initiative is more popular and successful than ever.

FDA’s breakthrough drug initiative is more popular and successful than ever.

The company has recalled one lot of 50% magnesium sulfate injection, USP due to particulates.

The agency issues safety guidance to minimize medication errors relating to product design and container closure design.

The FDA guidance describes information to provide to the agency when submitting a proposed proprietary name.

FDA added warning labels to medications containing saxagliptin and alogliptin after clinical trials linked the drugs to increased risk of hospitalization for heart failure.

The agency released a draft reflection paper on the extrapolation of data from adults to children in the development of pediatric drugs.

The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

Jack Lew, Obama’s secretary of the treasury, announced on April 4, 2016, that the US Department of the Treasury and the Internal Revenue Service (IRS) is issuing temporary and proposed regulations to limit the “benefits of and limit the number of corporate tax inversions.” The government bodies also plan to address earnings stripping in these inversions, so it will examine past inversion deals that have already been completed.

Even though the organizers of New York’s Tribeca Film Festival decided not to air the anti-vaccine film by discredited British researcher Andrew Wakefield, the so-called documentary is getting a healthy run and further perpetuating the myth of a link between childhood vaccination and autism.

The company received a positive recommendation for it’s Remicade biosimilar, Flixabi (infliximab).

The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.
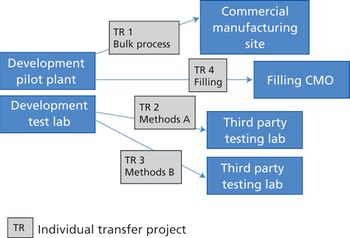
Careful planning, adequate staffing levels, and experienced project managers can help avoid pitfalls of transferring processes from one facility to another.

The European Medicines Agency report provides an analysis of the health-technology assessment pilot program, which began in 2010 and completed in March 2016.

The draft guidance states information concerning a clinical study of a biosimilar should only be included if it is necessary to demonstrate the safety and efficacy of the drug.
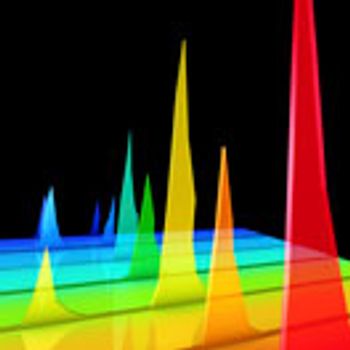
The authors provide application data to support the use of SEC beyond small-scale operations.

Global outbreaks energize vaccine R&D and drive production modernization.

The campaign against opioid abuse opens door to more innovative therapies.

Cinqair is approved for patients that have a history of severe asthma attacks despite receiving their current asthma medication.

The drug is marketed by Eli Lilly in the US, and will be available at the beginning of the second quarter of 2016.

The draft guidance outlines ways applicants can test for abuse deterrence in solid oral opioid drugs.

The agency announced enhanced warnings for immediate-release opioids to inform prescribers and patients of risks related to use.

Hospira recalls one lot of 8.4% Sodium Bicarbonate Injection, USP, due to particulate matter found within a single-dose glass fliptop vial.

The agency now allows production of water for injection by non-distillation technologies.

The agency cited Emcure Pharmaceuticals with CGMP violations.

Kovaltry is an unmodified, full-length recombinant factor VII product used to treat hemophilia A in adults and children.