
The Indian facility was cited for a range of quality and data integrity violations.

The Indian facility was cited for a range of quality and data integrity violations.

The agency published a guideline for the implementation of ICH Q3D.

FDA issued a warning letter to Lima & Pergher Industria e Comercio S/A for CGMP violations.

Regulatory agencies meet to discuss approaches to the development of antibacterial agents.

Airlines, airports, freight forwarders, and other cold-chain partners are taking a crash course in pharma cGMPs.
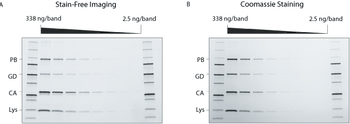
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.

Manufacturers and regulatory authorities seek coordinated lifecycle management policies.

FDA cites two Xinxiang Tuoxin Biochemical facilities with CGMP deviations.

Sandoz won FDA approval for its biosimilar version of Enbrel.

The guidance assists in the development, analysis, and presentation of microbiology data during antibacterial drug development.

The new guidance addresses FDA refuse-to-receive decisions in regards to impurity limits.

FDA issued a warning letter to the company for quality control violations.

The agency sent a warning letter to Cape Apothecary for adulterated drugs.

FDA issued a warning letter to College Pharmacy for multiple violations.

The Parenteral Drug Association develops program to address barriers to implementation of postapproval changes.

The debate over who has control over patents for CRISPR gene-editing technology came to a head on August 17, 2016 after an email was released from a former graduate student at the Broad Institute accusing Harvard-MIT of wrongfully securing patents to the technology.

The company is voluntarily recalling one lot of Oxacillin for Injection, USP, 10 g.

The Chinese facility was cited for data integrity violations.

Nine public health organizations submitted a letter to members of US House and Senate committees citing concerns with FDA’s decision to make changes to a section of the FDA and NIH Workforce Authorities Modernization Act.

The agency has adopted guidelines on the pharmacovigilance of biological drugs.

The agency released the GMP guidance to help manufacturers ensure accurate data and minimize risk.

FDA issued a warning letter to contract testing laboratory, Adamson Analytical Laboratories, for CGMP violations.

The company is voluntarily recalling one lot of product due to particulate matter.

The company is voluntarily recalling liquid products due to possible Burkholderia cepacia contamination.

The three-year report emphasizes the roll of collaboration in drug safety.