
Germany tops quality ranking in the inaugural CPhI Global Pharma Index.

Germany tops quality ranking in the inaugural CPhI Global Pharma Index.

As part of the deal, J&J’s Janssen will pay an upfront payment of $50 million to research, develop, and commercialize up to six bispecific antibodies.
The use of therapeutic vaccines presents a new way to manage diseases, such as cancer and sexually transmitted diseases.

ADC Bio experts warn of impending problems in the ADC pipeline with millions wasted in development costs.

The EMA’s Committee for Medicinal Products for Human Use has recommended marketing authorization approval for AstraZeneca’s benralizumab, a monoclonal antibody for treating severe asthma.

The company has introduced two new GMP vessel streams at its API manufacturing facility in Northumberland, UK that allows it to now offer fully integrated drug substance and drug product manufacturing.

The authors of the study believe it could have significant implications for the discovery of new dermatological products for major diseases such as psoriasis.

The company has officially opened a local brand office in South Korean to support its existing business in the region.

The agency gave Breakthrough Therapy Designation to GSK’s GSK2857916 monotherapy for the treatment of patients with multiple myeloma who have failed at least three prior lines of therapy.

The companies will collaborate to develop and manufacture Grid’s lead therapeutic candidate for the treatment of solid tumors.
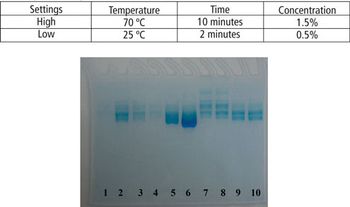
This article demonstrates that a modified SDS–PAGE can be easily used as a tool for quantifying the degree of protein degradation.

The contract research organization (CRO) has acquired Xenometrics, a non-clinical CRO specializing in preclinical assessment of new drug candidates.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.
Anna Kireieva/Shutterstock.comSpeed to market is essential in the biopharmaceutical industry today.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

As part of the $900-million deal, Incyte will pay $150 million upfront to develop and commercialize an anti-PD-1 drug candidate from biopharmaceutical company, MacroGenics.

AbbVie will pay a $205-million upfront payment and have the option to develop and commercialize two antibody targets globally.

The company has opened new facilities and an innovation center in Singapore, China that will focus on the development of plant-based products.

The collaboration aims to improve the pharmacokinetics of anti-C3 proteases developed by Catalyst to develop therapeutics for severe eye disorders.

Inovio has reported results from a study with non-human primates that showed 100% effectiveness with a DNA vaccine the company is developing with the US Army.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

The $72-million investment, part of a larger $850-million investment into its US operations, will allow the drugmaker to replace an outdated insulin vial-filling line and to upgrade technology at its Indianapolis manufacturing plant.

Through the support of the Japanese agency, Daiichi Sankyo intends to further develop its genetic vaccine platform focused around its new nucleic acid delivery technology.

The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.