
Researchers at the University of Illinois developed a method to transplant pancreatic islet cells from pigs more easily to treat type I diabetes.

Researchers at the University of Illinois developed a method to transplant pancreatic islet cells from pigs more easily to treat type I diabetes.

Ferring Pharmaceuticals will expand its biologics capabilities at its headquarters and manufacturing site in Saint-Prex, Switzerland.

A new report states that more research and clinical development must be done in the treatment of pain and addiction.

The acquisition strengthens an already existing collaboration between the companies to advance clinical development of therapies across multiple drug categories.

The companies will partner to further immuno-oncology research using humanized mice.

A collaboration between The Centre for Process Innovation and The Roslin Institute aims to develop commercially viable and scalable methods of producing biologics using transgenic animals.

The new Acquity Arc Bio System by Waters is specifically engineered to enable efficient transfer and improvement of bioseparation analytical methods.

The acquisition will strengthen Sanofi's R&D strategy and expands its franchise for rare blood disorders.

Corning will feature its 3D bioprinting technology and tools to support 3D cell culture at booth #1029 during the SLAS 2018 conference on Feb. 3-7, 2018 in San Diego, CA.

The funding from the Walloon Region will enable Univercells to start developing a manufacturing platform aimed at driving down costs of biosimilars manufacturing.

The acquisition strengthens Charles River Laboratories’ capabilities in the oncology and immunology therapeutic areas.

The acquisition, valued at EUR 520 million (US$631 million), would expand Takeda’s late-stage pipeline in gastroenterology and would extend an already existing collaboration between the two companies.

A Takeda and Denali collaboration includes three named programs for treating Alzheimer’s disease and other neurodegenerative diseases, using Denali’s antibody transport vehicle (ATV) technology to enhance blood-brain barrier penetration.

The companies aim to discover and develop locked nucleic acid oligonucleotides as orally available therapies for treating inflammatory bowel diseases.

The companies aim to develop a potential zinc finger protein transcription factor-based gene therapy for treating Lou Gehrig’s disease.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
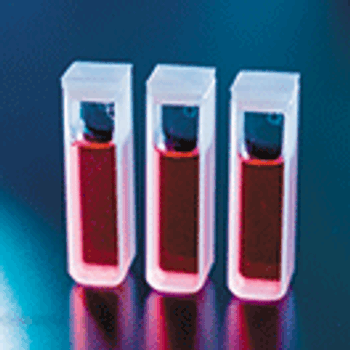
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

The company has completed development of a first-generation production process for its chimeric antigen receptor regulatory T cell product portfolio and is selecting a CMO for clinical supply.

Children with a rare brainstem-based cancer might be helped by a new immunotherapy that targets a mutated protein found exclusively in cancer cells.

Abzena has entered into a Master Services Agreement with a US biotech company to provide process development and manufacturing services to progress a novel antibody-drug conjugate (ADC) to clinical trials.

In partnership with Indian pharmaceutical firm, Torrent Pharmaceuticals, Novo Nordisk has expanded an insulin manufacturing facility at Torrent’s Indrad, Gujarat, India site.

The companies will co-develop and co-commercialize the lead candidate generated from their earlier collaboration to treat genetic blood disorders.

The new 30,000-L, $150-million biologics manufacturing facility in Wuxi, China, quintuples the company’s existing manufacturing capability.

The contract research, development, and manufacturing organization has expanded API aseptic manufacturing capacity at its Valladolid, Spain, facility.