
Follow-on versions of complex biologics require extensive expertise.

Follow-on versions of complex biologics require extensive expertise.

The pathway for biosimilar approval in the US has been set. But are US patients too far behind Europe?

An in-depth analysis of the patent provisions of the new legislation.

The third holy grail of biosimilars: interchangeability.
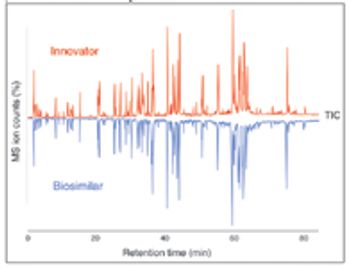
Intact protein LC–MS detected a mass variance of 62 Da and peptide mapping located a difference of two amino acids.

The new US legislation will forever alter the commercial landscape for biologics.

New R&D models must be tried, but it will take time to see if they work. In the meantime, a new kind of threat is on the horizon: biobetters.
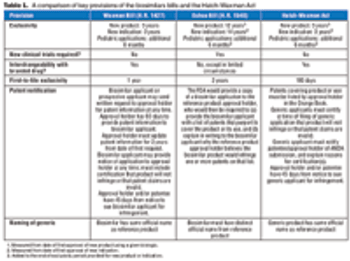
The introduction of two rival bills has intensified the long-simmering debate on biosimilars regulation in the US.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.
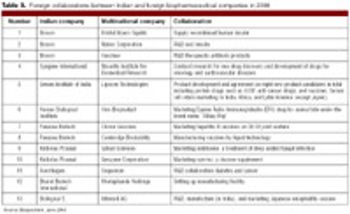
The new patent regime is challenging the Indian biopharm industry to transition from generics to novel products.

Did the 2005 Patents Act engender a Western intellectual property rights culture in the country?

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.

Both innovator and generics companies are using analytics to support comparability arguments.
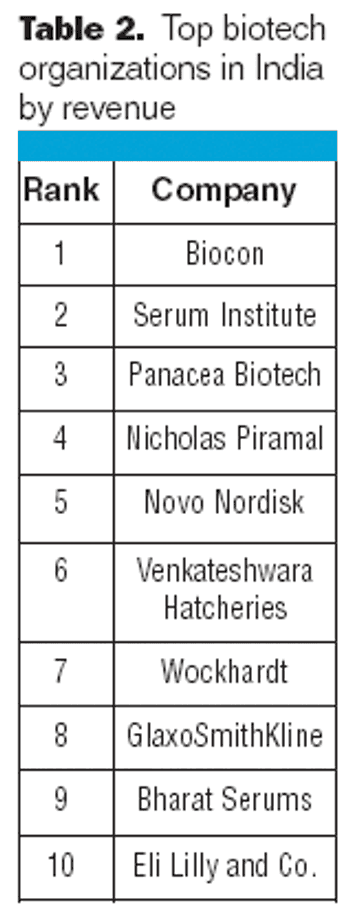
Indian biogenerics could form a major piece of the global biotherapeutics market in the future.

The industry needs a clear regulatory pathway for the approval of biosimilars.

Regulatory agencies have evolved along with the biotechnology industry to define quality standards.

The main testing and regulatory provisions of the FOB legislation reflect multiple trade-offs between the demands of innovators and generics firms.

In late June and early July, the US Congress moved forward on three important bills affecting the biopharmaceutical industry, related to follow-on biologics, the Prescription Drug User Fee Act (PDUFA), and the 2008 FDA budget.

The information provided by analytical testing is important in determining whether additional clinical trails are necessary to bring a follow-on to market.

The prospect of any cost savings has been fueling efforts to establish a pathway for follow-on biopharmaceuticals.

In the US, the patent term is 20 years from the filing date . . . commercial product may lose patent protection just as sales are ramping up.

Congress is not considering legislation that would expand FDA authority to regulate biologics.

Without generic competition, the US is at risk of losing its position of leadership in biopharmaceuticals.

Of all the protein products on the US market, at most 75 are likely to become genericized.