
Several groups, including pharmacies, call on FDA to preserve accepted International Nonproprietary Name conventions for biosimilars.

Several groups, including pharmacies, call on FDA to preserve accepted International Nonproprietary Name conventions for biosimilars.

A new report from GlobalData states that biosimilars will overtake the market share after 2019.

Draft guidance addresses the use of clinical pharmacology studies to determine biosimilarity of biologics.
The approval and acceptance of monoclonal antibody biosimilars is necessary if the biosimilars market is to experience real growth.

High technology assessments are having an impact on biosimilars development in Europe.

Automated sample handling, advanced glycan analysis and specially designed columns are helping biosimilar manufacturers speed up confirmation of the biosimilarity of their products.

Mike Jenkins, general manager of Catalent Biologics discusses the evolving landscape of the biologics market and hurdles faced in the development and manufacture of these innovative products.

As the complex requirements of manufacturing biologics are manifold, it is important that biomanufacturing companies adopt quality-by-design principles.
Review the importance of characterization studies during biosimilars development and related analytical methods.

The California State Assembly becomes the latest state to specify requirements allowing substitution of biosimilars.

FDA releases FY 2014 generic-drug user fees.

FDA funds research to further development of innovative generics, while working to address review and approval issues.

The EMA's Committee for Medicinal Products for Human Use has recommended granting of marketing authorizations for the first two monoclonal antibody biosimilars.

Global program seeks to confirm biosimilarity to support regulatory submissions in the US and EU.

Maryland is the latest state to consider whether to include additional requirements for substitution of biological products.

Sound policies are needed to govern the substitution of interchangeable biologics.

Development requirements and regulatory guidance for biosimilars and biobetters.

Manufacturing and in-depth characterization provide basis for demonstrating product equivalence.

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on mAbs.

AET BioTech and BioXpress Therapeutics have entered into an agreement to codevelop a biosimilar version of Abbott's tumor necrosis factor inhibitor monoclonal antibody adalimumab.
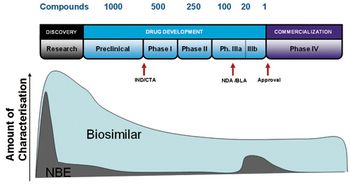
Understanding opportunities and challenges across all major phases of development.

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Does global development have to entail multiple comparability studies?

Has the long-awaited guidance answered all of the industry's questions?
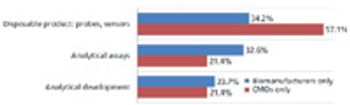
Biosimilar manufacturers need better expression systems and analytical tools to compete.