
Hospira’s Inflectra (infliximab), a biosimilar for Remicade, is approved by the European Commission.

Hospira’s Inflectra (infliximab), a biosimilar for Remicade, is approved by the European Commission.

The National Institute for Health and Care Excellence released an updated version of its biosimilar approach guidance, including increased consideration for technology appraisal, references in documentation, and the production of “evidence summaries”.

The Center for Drug Evaluation and Research seeks a more flexible system for assessing biotech product quality.

Pfenex announced that it entered into an agreement to partner with Hospira on the development and commercialization of PF582, a biosimilar candidate to Lucentis.

On February 3, 2015, the FDA published a notice in the Federal Register that it is soliciting input on the collection of data to support interchangeability claims in biosimilar applications.

Amgen announced that it met primary and secondary endpoints in its biosimilar evaluation of adalimumab for the treatment of rheumatoid arthritis, when compared to Humira.

The deal may offset billions of dollars in waning sales from Pfizer drugs slated to lose patent protection and provides Pfizer with a whole portfolio of biosimilar products.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.
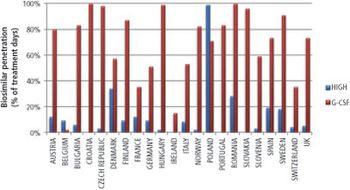
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.

Developing intellectual property standards for biological products is a point of conflict as negotiations on the Trans-Pacific Partnership continue.

Following a proposal by the World Health Organization, Australia will abandon a previously proposed update in biosimilar nomenclature.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

The move represents Hospira’s first biosimilar submission in the United States.

An FDA panel unanimously recommended the agency approve EP2006, Sandoz’s biosimilar for filgrastim.

FDA has scheduled a public meeting in early January to assess and weigh data on the first United States application for a biosimilar therapy.

NICE OKs biologics for the treatment of ulcerative colitis, changing its preliminary guidance that recommended against their use.

Sandoz announces their version of filgrastim, a follow-on biologic for the treatment of neutropenia, is as safe and effective as Amgen's Neupogen.

Enhanced R&D efforts and the growing manufacture of finished-dosage drugs in India will shape the country's future success, according to a new report from CPhI.

Ranbaxy and Epirus announce the launch of India's first biosimilar for Remicade.

Approximately 40% of total global growth will come from specialty medications, according to a new report from the IMS Institute for Healthcare Informatics.

Ralph G. Neas, president and CEO of the Generic Pharmaceutical Association, requests that Congress get involved in the support of a more competitive drug market.

A new report from The Pharmaceutical Care Management Association alleges that FDA hinders competition in the pharmaceutical industry and influences how a drug's price is calculated.

Amgen wants the regulatory agency to ensure biosimilar applicants follow the rules of the patent dispute resolution process delineated by the BPCIA.

Manufacturers face regulatory overhaul, while brand-generic debates escalate over biosimilars and labeling changes.

The final guidance explains some principles for developing biosimilars and establishes some rules about extrapolation across indications for various medical conditions.