
The collaboration will focus on differentiated cancer vaccine candidates in hematological and solid tumor indications to treat unmet medical needs.

The collaboration will focus on differentiated cancer vaccine candidates in hematological and solid tumor indications to treat unmet medical needs.

A tidal wave of questions floats the need for more upstream automation.

AstraZeneca has completed its acquisition of Icosavax, gaining the IVX-A12 vaccine candidate that targets RSV and hMPV.

Regulators from around the world will gather to help shape the mRNA and RNA therapeutics landscape at an exceptional April 2024 Washington, D.C. convocation.

Looking at the role that RSV and pediatric medical practices play in drug shortages.

Chris Spivey, editorial director for BioPharm International, discusses mRNA advances and the background for the leading scientific conference for the field, with host and co-organizer CureVac CEO Dr. Alexander Zehnder.

The new centralized hub will provide advanced testing of nucleic acids, which is expected to simplify mRNA substance testing.

Industry investments indicate increasing trust in the newest modalities, including mRNA approaches.

The final guidance document was issued to assist bio/pharma companies in the clinical development and licensure of COVID-19 vaccines.

The companies will co-promote GSK’s shingles vaccine, Shingrix, to healthcare professionals and points of vaccination in China.

The prize was awarded jointly to Katalin Karikó and Drew Weissman for their groundbreaking discovery regarding modification of the bases in mRNA.

Pfizer has entered into a collaboration with Ginkgo Bioworks to discover novel RNA molecules across priority research areas.

The companies plan on targeting the production of abundant, low-cost messenger RNA from C1-cells.
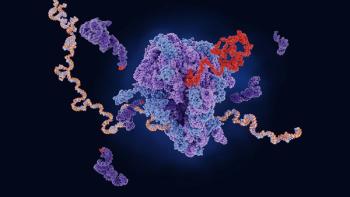
Despite its success, clinical and logistical roadblocks to mRNA cancer vaccine development remain.
Experts discuss the promise of personalized cancer vaccines through the inherent nimbleness of mRNA platform.

mRNA may be a modality whose chief advances are yet to come.

EMA looks to focus on mRNA vaccines because their classification depends on the target and/or whether they are obtained chemically or biologically.

The acquisition will continue to strengthen the area of conjugate vaccines and bioconjugate drugs and will expand Biosynth’s capabilities from good manufacturing practice facilities located in Berlin, Germany.

The new excipients manufacturing facility is expected to be completed in 2025.

Chris Spivey, editorial director, speaks with Sean Hart, CEO and SCO of LumaCyte, on percent transduction for AAV measured.

World leaders have set ambitious goals to respond more swiftly to the next pandemic, including the US goal to design, test, and review a new vaccine just 100 days after a pandemic declaration.

invoX Pharma has made a second tranche of investment in pHion Therapeutics to support further development of next-generation mRNA vaccines.

Vaccine nationalism strengthens viruses and poses deadly risks for all involved.

The data, published in the scientific journal Nature, demonstrated that half of patients developed T cells that could potentially fight pancreatic ductal adenocarcinoma.

Nicole Lewis, PVP Program Manager at Boston Children's Hospital, discusses the science behind precision vaccines, a form of personalized vaccines.