
Children with a rare brainstem-based cancer might be helped by a new immunotherapy that targets a mutated protein found exclusively in cancer cells.

Children with a rare brainstem-based cancer might be helped by a new immunotherapy that targets a mutated protein found exclusively in cancer cells.
The use of therapeutic vaccines presents a new way to manage diseases, such as cancer and sexually transmitted diseases.

This article explores some of the challenges, services, and technologies that go into ensuring that vaccines are properly temperature controlled and maintain product integrity for delivery to patients.
Challenge trials may increase in coming years as new and improved challenge agents better emulate “natural” disease states.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.
Despite the challenges and high cost of development, vaccine innovation is at an all-time high, as new approaches aim to improve global access.

The use of approved platform technologies can reduce the time and cost required to generate new vaccines.
This article discusses the potential of using high-productivity membrane chromatography to achieve intensified and flexible virus manufacturing.

The company expects to commercially launch the new vaccine in the US in the first quarter of 2018.

The Human Vaccines Project has created the Universal Influenza Vaccine Initiative, a research program that will aim to understand the human immune system’s role in the development of universal influenza vaccines.

The vaccine is approved for the prevention of shingles in adult patients aged 50 years and older.

Inovio has reported results from a study with non-human primates that showed 100% effectiveness with a DNA vaccine the company is developing with the US Army.

The companies aim to use CureVac’s proprietary messenger RNA technology to develop and commercialize up to five potential cancer vaccine products.

The two companies announced their collaboration for the production and development of cancer vaccines.

The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

Through the support of the Japanese agency, Daiichi Sankyo intends to further develop its genetic vaccine platform focused around its new nucleic acid delivery technology.

The company is investing EUR 170 million (US$201 million) to expand its vaccine manufacturing site in France for its newest influenza vaccine.

The collaboration will focus on developing a novel and differentiated challenge model for respiratory syncytial virus.

The Danish biotechnology company has been awarded a sole-source BARDA contract valued at more than $539 million for a freeze-dried smallpox vaccine.

Under Project BioShield, the agency could provide more than $170 million in funding to purchase and support late-stage development of Ebola vaccines and therapeutic drugs.

A grant from the Bill & Melinda Gates Foundation will advance PnuVax’s pneumonia vaccine’s clinical development and biomanufacturing scale-up using a low-cost manufacturing approach.

Under this partnership, Johnson & Johnson and BARDA will focus on the advanced development of a small-molecule drug and vaccine for the pandemic flu.
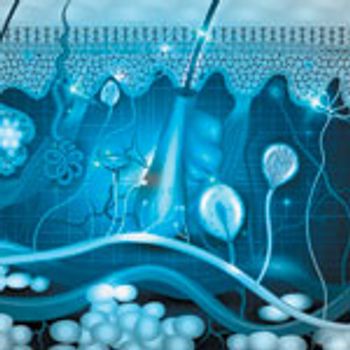
Using a hollow microstructured transdermal system to deliver vaccine directly to the dermis.

Shingrix represents a new, possibly better alternative to existing treatments.

The acquisition of Protein Sciences, a vaccines biotechnology company, strengthens Sanofi’s influenza vaccines portfolio.