
Scientists and industry experts seek effective preventive therapies to combat global disease.

Scientists and industry experts seek effective preventive therapies to combat global disease.

The pharmaceutical manufacturer pledged to freeze vaccine prices for Gavi-eligible countries for a decade.

Government and industry efforts to address manufacturing challenges move Ebola vaccine candidates into larger clinical trials.

Protein Sciences will evaluate sourcing Flublok from its Japanese licensee, UMN Pharma, which already runs a large-scale facility for the vaccine.

Baxter announced that it had entered into an agreement to sell its Vero cell technology and related assets to Nanotherapeutics.

US Department of Health and Human Services announced a declaration to provide immunity to legal claims made in the US in relation to three investigational Ebola vaccines.

Dalton Pharma Services announced it was awarded funding from ISTPCanada for its project to develop vaccines for respiratory syncytial virus and parainfluenza type 3.

The United States government is ramping up support for formulation, production, and packaging of Ebola treatments.

The agency recommends that companies developing drugs to treat Ebola apply for orphan drug designation.
Prefilled syringes offer advantages to manufacturers, healthcare professionals, and patients.

Demand for new therapies and vaccines spotlights production challenges.

Partnership is awarded for licensing of low-cost vaccine for the treatment of bacterial meningitis.

Novartis facility becomes the first US site licensed by the FDA to produce cell-culture influenza vaccines.

New formulations and expanded vaccine production are encouraged.

New approaches to vaccine production are targeting rapid supply for pandemic situations and broadly effective therapeutic treatments.
Review regulatory requirements and the use of viral-challenge studies in drug development.

HHS plan makes progress in ensuring availability of safe vaccines.

EMA releases an update on its flu vaccine guidance.

European Medicines Agency announces the launch of the Accelerated development of vaccine benefit-risk collaboration in Europe (ADVANCE) project.

PhRMA report reflects robust R&D in vaccine development.

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Creating an effective nucleic acid-based vaccine requires protecting the fragile nucleic acid from degradation, effective transfection of the targeted cells, and producing high enough levels of antigen to evoke a robust immune response.

Marco Chacon of Paragon Bioservices discusses the challenges associated with outsourced vaccine manufacturing.
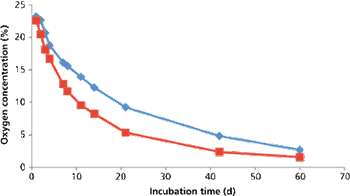
The authors describe a validation master plan for closed-vial filling technology.

Challenges of vaccine development include regulatory, technical, and manufacturing hurdles in translating a vaccine candidate into a commercial product.