
This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An understanding of global and national pharmacopoeias is crucial to understanding change processes and access to different markets.
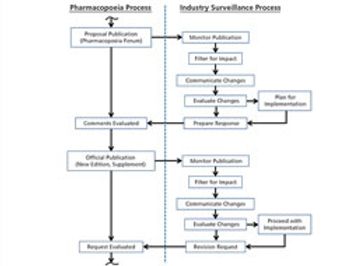
The process used to monitor and participate in pharmacopoeial changes is described.

Warning letters tell the tale of missteps by drug companies and offer a path to compliance for quality teams that monitor these enforcement actions.

Production and process controls, organization and personnel were the top problems found, while packaging and labeling citations increased in 2017 and 2018.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.

Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

Quality risk management plans provide identified actions to ensure a continuous supply of safe and effective drug products

According to a consent decree of permanent injunction issued in September 2019, the health and wellness companies must recall and stop distributing products until the companies comply with the Federal Food, Drug, and Cosmetic Act and other requirements included in the consent decree.

While labor and tariff reforms in the revised North American trade agreement may have more visible impacts on the United States economy, the final document levels a major blow to exclusivity and patent protections important to innovator biotech and pharma companies.

FDA has an official new leader, following a Senate vote to confirm Stephen Hahn as the agency’s next commissioner.

The biologic is a novel bone builder that has a dual effect of increasing bone formation and reducing bone loss.
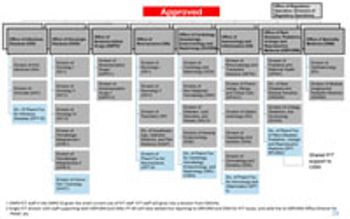
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.
Accelerated approval pathways and growing demand for cell and gene therapies are putting pressure on providers of cellular starting materials, and they must ensure a steady supply.

Investigating deviations or failures of combination products needs to accommodate both the drug and device components, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished

Problems in assuring reliable drug quality and supply dampens progress in bringing lifesaving therapies to market.

The agency sent a warning letter to Torrent Pharma after an inspection found violations of current good manufacturing practices that included a failure to thoroughly investigate batch failures.

The approval of Givlaari (givosiran) is the first for treatment of acute hepatic porphyria, which results in the buildup of toxic porphyrin molecules during the production of heme.

The agency’s approval of Abrilada (adalimumab-afzb), a biosimilar to Humira, brings the total of approved biosimilars to 25.

Roche’s ADC, Kadcyla, has been recommended for approval in the EU for for the adjuvant treatment of people with HER2-positive early breast cancer with residual invasive disease after neoadjuvant treatment.

Developers of biosimilars have become dismayed with difficulties in gaining acceptance and reimbursement from the US healthcare system.

In batch production, efficient exception management means reducing the time required to identify, review, and resolve process exceptions. Incorporating review by exception functionality within manufacturing execution system (MES) software can streamline biopharmaceutical product release.