
Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

With some FDA inspections on hold, will the US drug supply maintain its quality standards?

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article returns to the topic of complying with pharmacopoeial requirements with a case study at the intersection of monograph development and compliance.

In January 2020, the agency finalized six clinical development and manufacturing guidance documents and drafted new guidance on what would qualify new gene therapies as orphan drugs.

The agency is postponing the inspection of most foreign facilities through April 2020.

The action involves allowing specific National Institute for Occupational Safety and Health-approved respirators not regulated by FDA to be used in healthcare settings to increase the number of respirators available in the United States.

FDA issued a notice to drug compounders regarding the transition of licensure of biologics to the Public Health Service Act.

A guidance document answers questions regarding the transition of biologics applications from under the FD&C Act to the PHS Act.

The organization is postponing its annual meeting to July 2020 because of the developing COVID-19 outbreak.

Ensuring the quality of data in process monitoring and control systems starts in process development phases.

While new industry guidance documents issued by FDA speak to the agency’s efforts to promote the development of new gene therapies, certain hurdles remain to challenge stakeholders.

States, hospitals, and insurers support manufacturing arrangements to ensure access to affordable medicines.

Emergency actions to protect patients and the drug supply may have long-term implications.
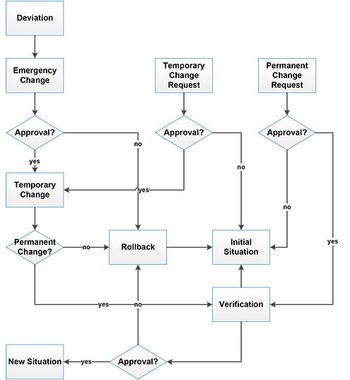
No matter why change may be needed, it is important to comply with all the relevant regulatory requirements, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

With the first announced drug shortage tied to the coronavirus outbreak, FDA emphasizes urgency for monitoring drug supply chain.

If approved, the therapy may become the first-choice treatment for relapsing multiple sclerosis patients and will be the first B-cell therapy that can be self-administered using an autoinjector pen.

As the date for transitioning the approval of biologic drug to a new pathway comes closer, FDA publishes a final rule and answers questions on the pathway changes.

FDA has granted breakthrough therapy designation to padcev (enfortumab vedotin-ejfv) in combination with Merck’s anti-PD-1 therapy keytruda (pembrolizumab).

US and European regulatory officials continue to anticipate supply shortages in multiple areas.

FDA’s Center for Biologics Evaluation and Research is planning on publishing nine specific guidance documents on gene therapies in 2020.

The agency is taking steps to monitor the supply chain and assist in the development of treatments.

The company has said that all three of its operating sites in China started back up on Feb. 12 and that it is closely monitoring the outbreak.

If approved, this treatment will be the first therapy targeted for METex14-mutated advanced lung cancer.