
The report highlights agency’s accomplishments in 2019, including its relocation to Amsterdam.

The report highlights agency’s accomplishments in 2019, including its relocation to Amsterdam.

Citing clinical trial data that hydroxychloroquine did not show benefits for COVID-19 patients, FDA removes emergency use authorization for the drug as a treatment for the novel coronavirus.

Will efforts to limit all returns on investment drive biopharma companies away from developing much-needed interventions for COVID-19.

The agency published guidance to clarify its enforcement of distribution of drug samples for drug marketing during COVID-19.

The 120-day pilot program was set up to help reduce the availability of unapproved opioids illegally offered for sale online.

The agency has added a COVID-19 innovation resource page and an education resources page to its website.

The European Commission is pledging EUR 300 million (US$339 million) to Gavi for vaccines for infectious diseases, in addition to an earlier pledge of more than EUR 1.5 billion (US$1.7 billion) made on May 4, 2020.

Johnson & Johnson’s Janssen Pharmaceutical Companies has received a positive opinion in Europe for its two-dose Ebola vaccine regimen.

Products must be manufactured in accordance with appropriate regulatory requirements, even during a pandemic, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

Despite the clear danger of COVID-19 to global health, vaccine opponents have gained ground, as fearful populations lose faith in the capabilities of industry and government to protect public health.

The complexity of biologics and the use of new technologies present challenges for complying with CGMPs.
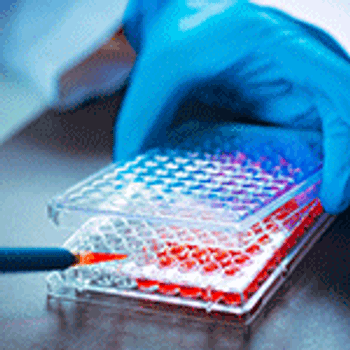
Advanced data analytics, including statistical modeling and machine learning technique, can enable more efficient and reliable bioprocesses.

Tools such as manufacturing execution systems, artificial intelligence, and software innovations are useful for enhancing data integrity protection.

Bioanalytical studies are an important aspect of biologic drug development that may necessitate partnering with bioanalysis experts.

FDA and the US Congress support innovation and access to cheaper medicines.

The White House looks to bring in credible experts for its Operation Warp Speed initiative to advance therapies and vaccines to combat the coronavirus pandemic.

The Trump Administration has awarded a hefty contract to a new pharmaceutical manufacturing consortium to produce in the United States all components of certain critical medicines needed to combat COVID-19.

Biopharmaceutical companies and federal agencies have been working overtime and assuming considerable risk to be able to supply billions of doses of any safe and effective preventive.

The International Coalition of Medicines Regulatory Authorities held a virtual meeting of regulators from around the world to discuss policy issues and regulatory requirements to respond to the ongoing COVID-19 pandemic.

The EU Executive Steering Group on Shortages of Medicines Caused by Major Events met on May 13, 2020 to discuss measures the European Union is taking to ensure the availability of medicines during the COVID-19 pandemic.

The European Medicines Agency is emphasizing the need for the research community to pool resources to determine which COVID-19 treatments and preventions are safe and effective.

A temporary restraining order was entered against Xephyr LLC, doing business as N-Ergetics requiring the company to immediately stop distributing colloidal silver products.

Some observers fear that political interference in the process may erode confidence in the scientific basis for FDA regulatory decisions.

The agency is responding to companies promoting and selling medical products that claim, but are unproven, to prevent or treat COVID-19.

AbbVie and Allergan have satisfied all required antitrust clearances for the acquisition.