
EMD Millipore will provide process development services for Precision Biologics’ preclinical monoclonal antibody.

EMD Millipore will provide process development services for Precision Biologics’ preclinical monoclonal antibody.

They may not be glamorous, but buffers play an important role in biopharma manufacturing.
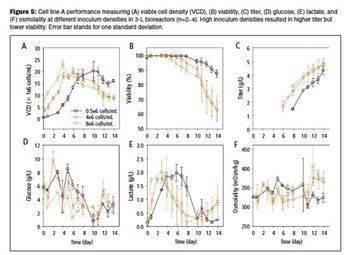
The use of commercially available media to achieve high titer in early process development is discussed.

What the industry's future holds and what needs to be done to get there.

NIBRT's Ian Nelligan on what to expect when starting an upstream process, including the choice between single-use and stainless-steel bioreactors.

A technical rountable featuring Sartorius Stedim Biotech, Pall Life Sciences, 3M Purification, Asahi Kasei Bioprocess, and Bio-Rad Laboratories.
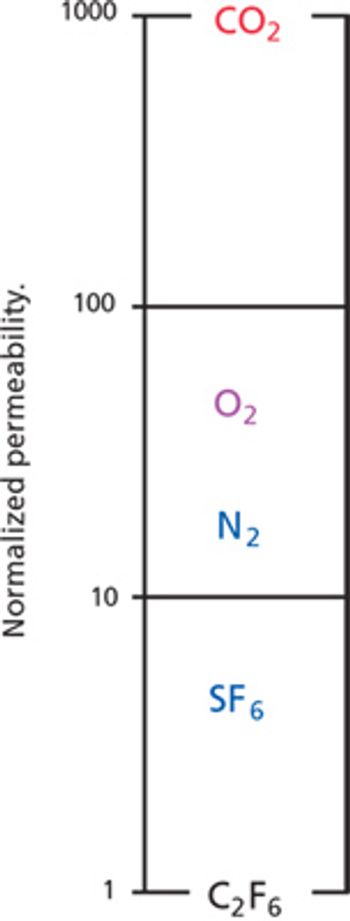
The authors developed a test for defects in filter membranes.
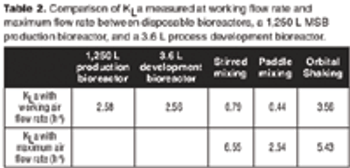
A case study to compare the performances of several types of mixing in disposable bags with stainless steel bioreactors.

These new analytical methods can reduce analysis and product development time.
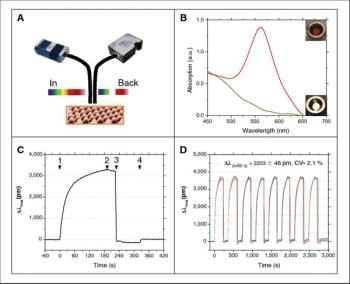
Use it label-free, or add labels to detect contaminants in solution.
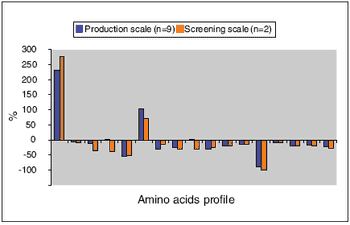
Rolling-tube system balances scale-up accuracy and thoroughput.

Identify the best experimentation methods for the data you need.
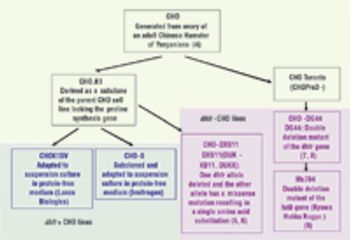

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.