
Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

Managing and prioritizing risk is essential to ensuring raw material quality. USP is developing new guidelines to make the work easier.

This article provides an overview on important aspects related to bracketing strategies in Japan.

As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
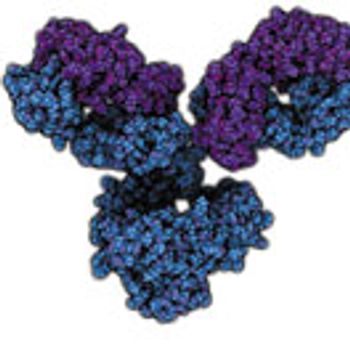
The objective of this study was to assess the impact of manufacturing-scale, freeze-thaw conditions on aggregation and subvisible particle formation of a monoclonal antibody solution (mAb-A; IgG1) using a small-scale model.

Excipient selection strongly influences lyophilization performance for biologic drugs.

High-purity low-endotoxin sugars improve robustness and stability of protein formulation and improve drug product quality.
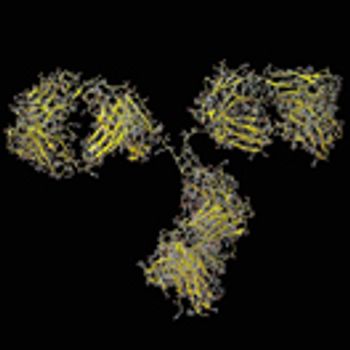
An approach to stabilize PBS-based formulations could provide a simple physiological solution for use of proteins in research, preclinical, diagnostics, and clinical studies, as well as commercial biotherapeutic products.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?
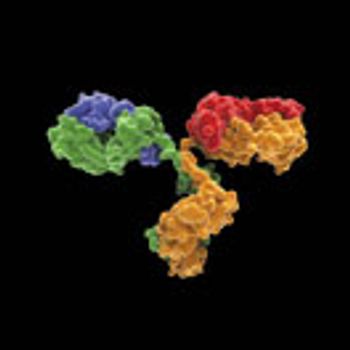
The study demonstrates a systematic approach to stabilize PBS-formulated mAbs against freeze-thaw degradation.
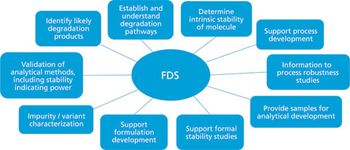
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.

A new study in Nature Communications explores how to remove the bulk of the soaps that are added to injectables to make hydrophobic drugs more soluble.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

The company presented a portfolio of new products during the meeting in Madrid.
When using media supplements in biologics, it is important to have a key understanding of both the supplement and the base medium to ensure high titer and stability.

Ethylene vinyl acetate (EVA) drug-release technologies are explored.

The report highlights a need for greater third party certification to ensure GMP vigilance.
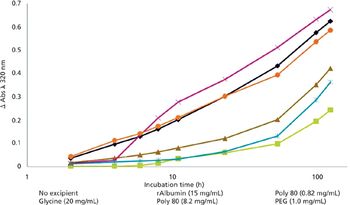
Recombinant albumin can stabilize a drug product and assist in API release.
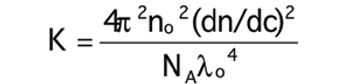
Can increase in ionic strength result in higher viscosity?