
A new study concluded although some mAb products have heterogeneous variants, these charge variants are associated with similar potency and pharmacodynamic profiles as originator molecules.

A new study concluded although some mAb products have heterogeneous variants, these charge variants are associated with similar potency and pharmacodynamic profiles as originator molecules.
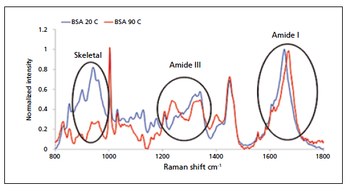
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

Expectations are high for rapid testing methods, but demonstration of comparability proves challenging.
Advances in glycan analysis are enhancing biologics development and quality control processes.

Whether taking an upstream, downstream or holistic approach, there are many factors to consider when choosing viral clearance methods.
The authors present a review of the techniques commonly used for glycosylation analysis.
Dynamic light scattering techniques can monitor viruses and virus-like particles in their native state.

Three case studies illustrate some analytical methods important for stability testing.
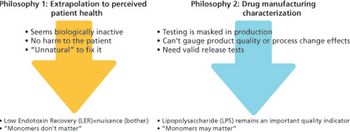
Low endotoxin recovery represents an opportunity to add value to the characterization of biologic drug products.

This article reviews factors that affect protein stability at different steps of the product manufacturing process and strategies to minimize their impact on product quality.
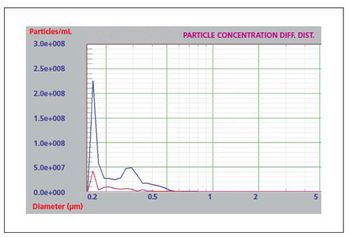
Liquid particle counters are ideal for protein aggregation studies.
The thorough analysis of a therapeutic protein product’s propensity to aggregate may be a necessary step in the prevention of a cell-mediated immune response.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.
Ligand-binding assays are fundamental to characterizing biosimilars.

Development and validation of two pivotal assays for in-vitro comparability testing of insulin biosimilars.

An automated analytical method determines the purity of chromogenic, fluorescently tagged proteins or metal-bound proteins.
Light scattering analysis combined with more rapid size exclusion chromatography improves protein characterization.
USP evaluates raw materials used in the chemical synthesis of peptides.

Aggregation System Improves Analysis of Sub-Visible Particles
A well-designed comparability study can demonstrate the performance and advantages that can be gained when adopting a new protocol.
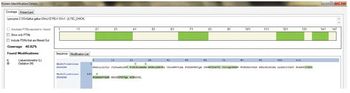
A simple, sensitive, and universal platform for identification and quantification of trace-level protein impurities in biotherapeutics is described.

A proposed new guideline provides a global policy for limiting metal impurities qualitatively and quantitatively in drug products and ingredients.

NIBRT's Pauline Rudd on what to expect when performing glycan analysis.
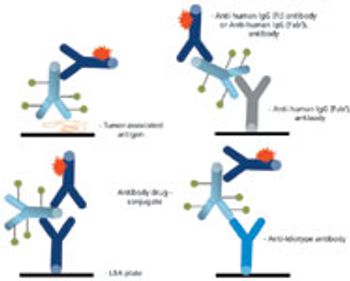
The complex structure of ADCs necessitates different analytical strategies than those for either small molecules or unconjugated monoclonal antibodies.
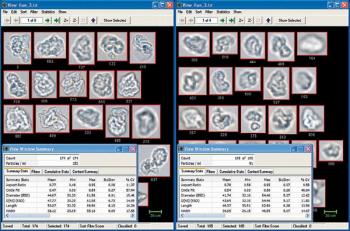
Overcoming limitations of volumetric techniques and detecting transparent particles.