
Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.

Challenging molecules and markets are driving the development of new solutions for drug delivery.

Agilent Technologies announces plans to build a new oligo manufacturing facility in Colorado that will double current capacity.

Technology advances enable contract service providers to keep pace with the demands of existing and emerging biologic-based therapies.

For successful drug delivery partnerships, it’s important to take a long-term view, focus on simple designs, and address potential payer concerns up front.

The company’s method reduces the time required to crystallize antibodies from weeks to one day.

The company plans to reformulate injectable products to make them into inhaled and intranasal medications.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.

Catalent’s licensing of Excelimmune’s antibody combination therapy platform can enable more consistent, cost effective production of antibody combinations.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

The report highlights a need for greater third party certification to ensure GMP vigilance.

The facility, which includes state-of-the-art formulation, analytical and synthetic laboratories as well as a customer training center, will focus on bioavailability enhancement and oral dosage formulations.

Stephen Taylor PhD, vice-president and commercial director at Fujifilm Diosynth Biotechnologies, addresses some of the challenges facing biologics outsourcing.
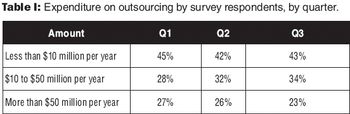
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.