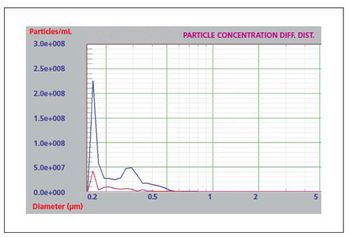
Liquid particle counters are ideal for protein aggregation studies.

Liquid particle counters are ideal for protein aggregation studies.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.

Clinical trial results suggest that monoclonal antibodies targeting the function of proinflammatory cytokine IL-17A in psoriasis may be significantly superior to other treatments.

Early access to Merck’s Keytruda in the United Kingdom is granted under the UK’s Early Access to Medicines Scheme.

FDA approved Sandoz’s Zarxio (filgrastim-sndz) on March 6, 2015. The approval is a groundbreaking decision, as Sandoz is the first pharmaceutical company to have a biosimilar product approved in the United States. Known as Zarzio outside of the US, Sandoz says its biosimilar filgrastim is already available in more than 60 countries worldwide, has generated more than 7.5 million patient-days of exposure, and is "the most widely used filgrastim in Europe."
The thorough analysis of a therapeutic protein product’s propensity to aggregate may be a necessary step in the prevention of a cell-mediated immune response.

Remsima will now be available for patients in 12 additional countries in the European Union.

Actavis says it plans to use the Allergan corporate name for its branded products.
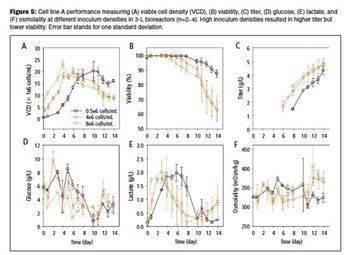
The use of commercially available media to achieve high titer in early process development is discussed.

Following a proposal by the World Health Organization, Australia will abandon a previously proposed update in biosimilar nomenclature.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

Xencor's technology focuses on the use of antibody and protein biotherapeutics to treat immune-related diseases.

Tosoh Bioscience’s CaPure-HA hydroxyapatite chromatography resin is designed for the purification of monoclonal antibodies, polyclonal antibodies, and antibody fragments, and the separation of antibody isoforms and isozymes.

Eppendorf's Cell Culture Consumables have an ISO class/GMP class C clean room production standard.

The Apollo platform can be used throughout a product?s entire lifecycle.

The EZ-Fit Filtration Unit, a ready-to-use, disposable filtration device for bioburden testing from EMD Millipore, the Life Science division of Merck KGaA, increases reliability and streamlines workflows.

TOC Analyzer Increases Productivity
Handheld Analyzer Improves Accuracy

SEC-MALS Detector for UHPLC

Clinical Syringe Packages Increase Productivity

Integrated Single-Use Sterile and SIP Connector Provide Flexibility

Aseptic Valve Range Increases Sterility

The ?DAWN can be attached to any UHPLC system to determine molecular weights and sizes of proteins.

Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

OPUS technology designed to process large single-use bioreactor harvests.