
Cellexus Biosystems (Cambridgeshire, UK, www.cellexusbiosystems.com) has selected Julie Bick, MD, as its chief scientific officer.

Cellexus Biosystems (Cambridgeshire, UK, www.cellexusbiosystems.com) has selected Julie Bick, MD, as its chief scientific officer.

The FDA has licensed Baxter Healthcare's (Deerfield, IL, www.baxter.com) Ceprotin, the first biologic treatment for patients with a rare genetic defect that can cause a potentially life-threatening clotting disorder.

Abbott (Abbott Park, IL, www.abbott.com) recently announced the official opening of its new biologics manufacturing facility in Puerto Rico to support the long-term supply of its leading biologic agent, HUMIRA (adalimumab), and other future biologics.

Singapore's efforts to grow its biologics manufacturing sector received a significant boost on March 28, 2007, when the Singapore Economic Development Board (EDB) announced that Genentech, Inc. (San Francisco, CA, www.gene.com) has decided to establish a commercial-scale microbial-based biologics manufacturing facility in Singapore.

Joanne Less has become the acting director of the US Food and Drug Administration's Office of Combination Products (OCP).
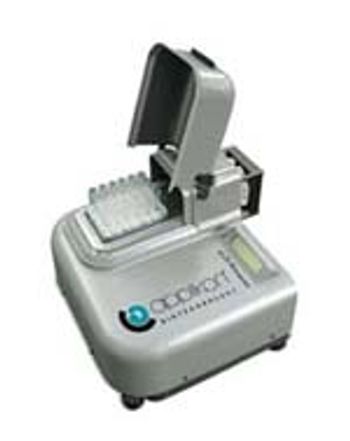
The µ24 bioreactor from Applikon (www.applikon.com) offers a platform for fermentation in a very small volume.

Patients receiving palliative care in hospices, hospitals, and other settings can benefit from a subcutaneous injection of morphine with Hylenex recombinant (hyaluronidase human injection).

New Brunswick Scientific (www.nbsc.com) has introduced a compact benchtop cell culture bioreactor capable of regulating 32 process loops per vessel, and up to four vessels simultaneously, including integrated control of multiple sensors, scales, analyzers or other external devices.

Nautilus Biotech (Evry, France, www.nautilusbiotech.com) has signed a collaboration and license agreement with HanAll Pharmaceutical (Seoul, South Korea, www.hanall.co.kr) to develop and commercialize three Nautilus Biotech products in South Korea: Belerofon (Interferon alpha); Vitatropin (human growth hormone); and EporalTM (erythropoietin).

Timothy Stitely will become FDA's chief information officer (CIO) effective March 5, 2007.

The biopharmaceutical industry has gained a lot of experience in monitoring glycosylation, but still has a lot to learn about the structure–function relationship.

Following a series of recent setbacks, Acambis plc (Cambridge, UK, www.acambis.com) has initiated a restructuring, aimed at increasing the focus of its resources on key programs and core operational capabilities, and significantly lowering its cost base.

Cities, counties, states; every political entity in our country is driving to develop a biotechnology presence in its respective region.

An FDA advisory panel has recommended that the agency approve Sanofi-Pasteur's (Lyon, France, www.sanofipasteur.com) H5N1 bird flu vaccine.

The US Food and Drug Administration (Rockville, MA, www.fda.gov) commemorated its 100th anniversary in 2006 as America's premier public health agency aimed at protecting and promoting public health.

The Biotechnology Industry Organization (BIO, Washington, DC, www.bio.org) has elected Pfizer (New York, NY, www.pfizer.com) Chairman and CEO Jeffrey B. Kindler to BIO's board of directors.

Cougar Biotechnology, Inc. (Los Angeles, CA, www.cougarbiotechnology.com), has appointed Richard B. Phillips, PhD, as VP of regulatory affairs and quality assurance.

GlaxoSmithKline (London, UK, www.gsk.com) has announced clinical trial data from two new studies, which show that its pre-pandemic flu vaccine, formulated with GSK's proprietary adjuvant system, works against diverse strains of H5N1.

The United States Pharmacopeia (USP, Rockville, MD, www.usp.org) and the UK's National Institute for Biological Standards and Control (NIBSC, Hertfordshire, UK, www.nibsc.ac.uk) are seeking participants in a study of analytical methods used by the industry to characterize and quantify oligosaccharides.

BioCryst Pharmaceuticals, Inc. (Birmingham, AL, www.biocryst.com) and Shionogi & Co., Ltd. (Osaka, Japan, www.shionogi.co.jp) have signed an exclusive license agreement to develop and commercialize BioCryst's lead influenza neuraminidase inhibitor, peramivir, in Japan for the treatment of seasonal and potentially life-threatening human influenza.

Vasogen, Inc. (Ontario, Canada, www.vasogen.com), has appointed Terrance H. Gregg to succeed David G. Elsley as president and CEO of Vasogen.

Cell Therapeutics, Inc. (CTI, Seattle, WA, www.ctiseattle.com), has formed a new spin-off company, Aequus BioPharma, Inc., to develop a novel process to extend the half-life of proteins.

Sartorius AG (Goettingen, Germany, www.sartorius.com) has acquired a substantial stake in the biopharmaceutical supplier Stedim Biosystems S.A (Aubagne, France, www.stedim.com) and combined its biotechnology division with Stedim.

The Group of Seven (G7) industrialized nations plans to sign an agreement to provide $1.5 billion dollars to develop vaccines for diseases including HIV/AIDS, tuberculosis, and malaria that largely affect developing countries, reported Reuters on February 6, 2007.

Laureate Pharma (Princeton, NJ, www.laureatepharma.com) has signed an agreement with Boehringer Ingelheim (BI, Ingelheim, Germany, www.boehringer-ingelheim.com) for access to BI's comprehensive manufacturing technology platform for biopharmaceutical products produced by mammalian cell culture.

Manufacturers of vaccines used to prevent potential avian pandemics will receive immunity from product liability claims, HHS announced on January 1.

Waters Synapt High Definition MS System from Waters Corporation (www.waters.com) is designed for researchers working at the limits of conventional mass spectrometry and who need to further characterize and define complex samples.

A new ?9.5 million R&D program has been launched by ITI Life Sciences (Dundee, Scotland, www.itilifesciences.com) to develop an automated process to produce high-quality human stem cells.

Wyeth Pharmaceuticals, a division of Wyeth (Madison, NJ, www.wyeth.com), has signed agreements with Nautilus Biotech (Evry, France, www.nautilusbiotech.com) and MediVas LLC (San Diego, CA, www.medivas.com), a biomaterials company specializing in improved delivery of biologics.

The Bush administration's spending plan for 2008 treats the US Food and Drug Administration (Rockville, MD, www.fda.org) fairly well, considering the cuts directed at Medicare, Medicaid, and children's health programs and flat funding for the National Institutes of Health (NIH).