
The challenges and strategies of assessing and mitigating risk in biopharmaceutical manufacturing are discussed.

The challenges and strategies of assessing and mitigating risk in biopharmaceutical manufacturing are discussed.

The use of single-use systems in downstream processing offers benefits in filtration and sampling and may reduce the risk of contamination.


Advances in adventitious agent detection methodology are bringing benefits, but more work needs to be done.
When using media supplements in biologics, it is important to have a key understanding of both the supplement and the base medium to ensure high titer and stability.
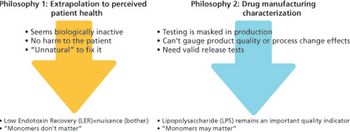
Low endotoxin recovery represents an opportunity to add value to the characterization of biologic drug products.

The European Union has a challenging task ahead as it strives to harmonize regulations on advanced therapy medicinal products.

As biopharma enjoys success, it cannot ignore pressing patient access questions.
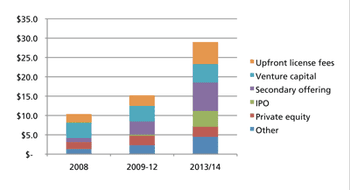
While all market signs are pointing up, memories of past setbacks may discourage CDMOs from expanding capacity.

Manufacturers and FDA look for innovative strategies to meet accelerated timeframes.
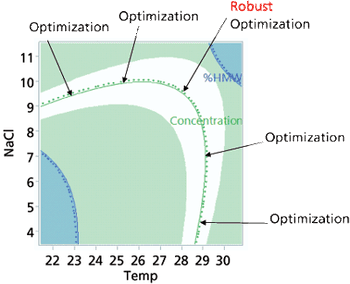
Approaches to the generation of process models, optimization techniques, and application of a design space are explored.

GEA’s Panther NS3006L high-pressure homogenizer is a standalone unit designed to reduce particle size to nanometer ranges.

Sartorius Stedim Biotech’s Sartoclear Dynamics is a clarification system designed to harvest mammalian cell cultures with high cell densities using single-use technology.

Click the title above to open the BioPharm International July 2015 issue in an interactive PDF format.