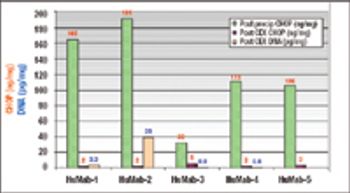
Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.

Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.

How to implement a risk-based approach to eliminate viruses using orthogonal technologies.
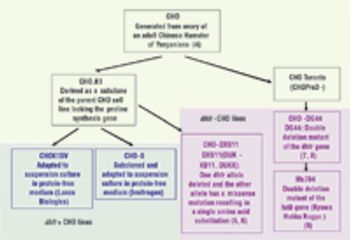

Interview with Roger Lias, president and group commercial director at Eden Biodesign, Inc.

How to implement a risk-based approach to eliminating viruses.
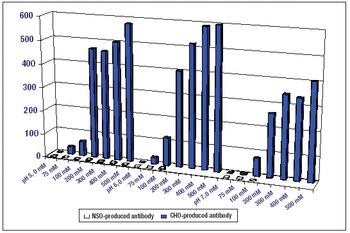
If certain engineering challenges can be addressed, precipitation may prove to be a valuable tool for antibody purification.

Although contaminants and other parameters may be main causes of filter breakdown, some nanofilters still remove viruses at high Log Reduction Value (LRV).

The HSV-1 and HVP-2 titers were determined by the inoculation of test solutions into Vero cell cultures and calculated using the Reed M?ench method.
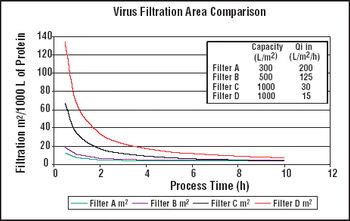
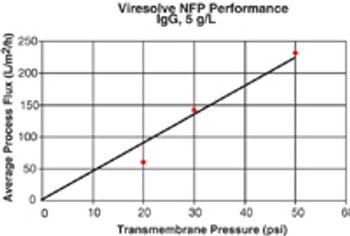
It is important to ensure that flow decay during processing is comparable to that observed during retention studies.
Human plasma provides a rich source of therapeutic medicines, including gamma globulins, coagulation factors, albumin, alpha anti-trypsin, and others. In 2001, sales of immuno gamma-globulin (IgG) were estimated at $2 billion with a production rate of 50 metric tons for the year.1 A number of new therapeutic products have recently been introduced including Gammimune from Bayer, RhoPhylac from ZLB Behring, and Octagam from Octapharma.